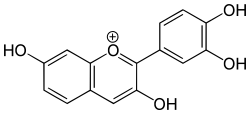
Fisetinidin is an anthocyanidin. It has been obtained from the heartwood of Acacia mearnsii,[1] from the bark of Rhizophora apiculata[2] and can also be synthesized.[3] Fisetinidin is very similar in structure to fisetin,[3] which itself differs in structure from quercetin only by an additional hydroxyl group on the latter.
| |
| Names | |
|---|---|
| IUPAC name
2-(3,4-dihydroxyphenyl)chromenylium-3,7-diol chloride
| |
| Other names
Fisetinidin chloride
3,3',4',7-Tetrahydroxyflavylium chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H11O5+ (Cl−) | |
| Molar mass | 306.69 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
An assay of twenty flavonoids showed fisetinidin to be the least effective in inhibition of CD38 enzyme.[4]
Tannins
editFisetinidin can compose tannins.[1] The polymers are then called profisetinidin (Porter, 1992).[2]
See also
editReferences
edit- ^ a b D. G. Roux; E. Paulus (February 1962). "Condensed tannins. 12. Polymeric leuco-fisetinidin tannins from the heartwood of Acacia mearnsii". Biochem. J. 82 (2): 320–324. doi:10.1042/bj0820320. PMC 1243455. PMID 14494576.
- ^ a b Afidah A. Rahim; Emmanuel Rocca; Jean Steinmetz; M. Jain Kassim; M. Sani Ibrahim; Hasnah Osman (2008). "Antioxidant activities of mangrove Rhizophora apiculata bark extracts". Food Chemistry. 107 (1): 200–207. doi:10.1016/j.foodchem.2007.08.005.
- ^ a b M. Gábor; E. EperJessy (10 December 1966). "Antibacterial Effect of Fisetin and Fisetinidin". Nature. 212 (1273): 1273. doi:10.1038/2121273a0. PMID 21090477. S2CID 4262402.
- ^ Kellenberger E, Kuhn I, Schuber F, Muller-Steffner H (2011). "Flavonoids as inhibitors of human CD38". Bioorganic & Medicinal Chemistry Letters. 21 (13): 3939–3942. doi:10.1016/j.bmcl.2011.05.022. PMID 21641214.