| Submission declined on 19 April 2024 by Stuartyeates (talk). This draft's references do not show that the subject qualifies for a Wikipedia article. In summary, the draft needs multiple published sources that are:
Where to get help
How to improve a draft
You can also browse Wikipedia:Featured articles and Wikipedia:Good articles to find examples of Wikipedia's best writing on topics similar to your proposed article. Improving your odds of a speedy review To improve your odds of a faster review, tag your draft with relevant WikiProject tags using the button below. This will let reviewers know a new draft has been submitted in their area of interest. For instance, if you wrote about a female astronomer, you would want to add the Biography, Astronomy, and Women scientists tags. Editor resources
|  |
 Comment: Tone is wrong; most refs aren't secondary. Stuartyeates (talk) 20:13, 19 April 2024 (UTC)
Comment: Tone is wrong; most refs aren't secondary. Stuartyeates (talk) 20:13, 19 April 2024 (UTC)
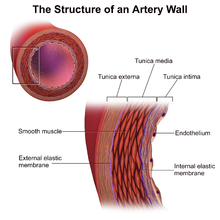
Perivascular administration concerns drug delivery directly to the adventitia of a blood vessel. Perivascular delivery is a distinct subset of pharmacological administration and aims to locally release pharmaceuticals to the outside of a blood vessel. Perivascular drug administration is a broad field of drug delivery that includes various types of drug application to the many perivascular spaces in the body, most specifically administration to the Tunica Externa (sometimes called Tunica Adventitia) , methods of perivascular administration have been proposed for treating various complications following vascular surgery, especially Neointimal Hyperplasia,[1] Restenosis[2] and reducing the likeliness of vein graft failure.[3] Additionally, perivascular administration has been explored to help reduce intimal hyperplasia secondary to arteriovenous fistula creation for dialysis[1].
Breakdown of system morphologies edit
Perivascular Drug delivery systems are defined by both their mechanical properties and the drug release kinetics facilitated by the system morphologies. Different system morphologies have different benefits and limitations compared to one another.[4] Various types of system morphologies are defined below.
Gel edit
Gel based perivascular drug delivery techniques are one of the most researched perivascular systems, the first perivascular systems were also gel based.[5] Gel systems are defined by being semi-solid and do not maintain a defined shape. Gel systems have easily tunable viscosities, are the least invasive of all perivascular administration systems, and can be injected even without full vascular surgery. Having the greatest ease of administration of any perivascular system.[6] Gels can also have various advanced drug delivery techniques (nanoparticles, degradation-controlled, etc...) implanted into the gel to allow great amounts of control over drug release kinetics. However, gels also have severely lacking vasoconstriction, do not provide any beneficial material properties, and are lacking localization as injected gels can move from the target site due to vascular resistance.[4][7]
Gel based systems offer many advantages over other perivascular administration techniques, large amounts of research has been done to attempt to improve the lacking localization and material properties which holds back gel-based techniques. A few notable examples of this research include a Poloxamer (a triblock copolymer) gel which has scene multiple preclinical studies which gelates when pushed beyond a tunable temperature, this system provides the ability to be injectable as a liquid but gels at body temperature, systems have been proposed utilizing this technology for the treatment of Neointimal Hyperplasia.[8] Regel® a triblock PEG-PLGA-PEG copolymer which gelates on exposure to water provides similar localization benefits in the treatment of neointimal hyperplasia.[9] Hyaluronic acid (HA) gels are also proposed as possible options for neointimal hyperplasia treatments, as cross-linked HA gels exhibit membrane adhesive properties which could improve localization.[10] Dopamine has also been added to HA gels to improve vascular adhesion.[11] Additionally, research suggests that presence of high concentrations of HA could suppress cellular proliferation, possibly providing possible patient outcomes for Neointimal Hyperplasia treatment.[12]
Mesh edit
Mesh systems defined by being a macroporous solid which holds its shape.[4] These mesh systems are the polar opposite of Gel based systems, meshes provide exceptional localization and mechanical properties, but are highly invasive, require specialized equipment and utilize expensive procedures to implant.[13] Additionally, while meshes have been coated to provide bolus drug, the morphology of a Mesh does not yield itself well to sustained drug release, and no sustained release perivascular mesh has been researched as of yet.[4]
The makeup of the mesh system impacts its material properties, the most popular types of meshes are either metal or polymeric, and are woven, knitted, or braided.[14] The choice of mesh design impact pulse compliance and flexibility.[15] A few examples of relevant research in the design of Perivascular Meshes include non-constrictive PET polymeric meshes for Neointimal Hyperplasia treatment,[16] a constrictive knitted nitinol mesh that exhibits a 60% reduction in Neointimal Hyperplasia,[15] and a constrictive braided nitinol mesh which exhibits a 91% reduction in presentation of Neointimal Hyperplasia symptoms.[17] Mesh based systems have seen extensive research, however as time passes data shows that while the mechanical properties of implantable meshes are valuable for promoting revascularization drug delivery is a more impactful aspect, thus the lack of sustained release in Mesh systems holds them back.[4]
Sheath edit
Sheath systems are solid, shape holding, microporous, tubular implants with diameters of less than 200 microns.[4] Sheaths are similar to Mesh systems in that they are very difficult to implant, requiring expensive and highly invasive surgery as well as specialized equipment. Unlike meshes however, sheaths are capable of sustaining drug release over a longer period of time, trading a minor decrease in material properties (sheaths are less capable of vasoconstriction) for the ability to sustain drug release allows sheaths to serve as an upgrade over traditional mesh systems in many situations.[4]
A few examples of sheath based perivascular systems include a failed clinical trial of an un-constrictive Dacron® sheath fitted to Polytetrafluoroethylene (PTFE) ribs, which failed to improve patient outcomes due to increased risk of thrombosis, thought to be caused by excessive rigidity.[18] An un-constrictive PTFE sheath which showed a 72% decrease in risk of hyperplasia in vivo.[16] A constrictive collagen sheath showed a 45% decrease in hyperplasia risk.[19] Additionally, sheath based sustained release drug delivery systems showed possibilities of hyperplasia risk suppression, with a polycaprolactone (PCL) + Poly (ethylene glycol) methyl ether (mPEG) sheath which elutes Paclitaxel.[20]
Cuff edit
Cuff systems are solid, shape holding, microporous, non-tubular implants with diameters of less than 200 microns, that are sutured or sealed.[4] These cuff-based systems have superior ease of installation compared to sheaths and meshes, as the cuff is essentially a wrap which is sinched around the vein location. Cuffs have the added benefit over wraps of being able to apply variable amounts of vasoconstriction based on the cinch force provided by the physician. Cuffs have slightly lacking localization when compared to sheaths or meshes but are much easier to install, additionally cuffs are capable of sustained drug release.[20] The most important aspect for cuffs and wraps is the manipulation of flexibility.[10]
Examples of cuff-based perivascular systems include a plasticized PEG based wrap which attempts to improve elasticity for hyperplasia treatment,[21] a flexible vinyl acetate (EVAc) cuff,[22] PLCL cuffs to provide high fracture strain,[23] there are also examples of drug eluting cuffs which elute drugs such as verapamil[24] to reduce chances of thrombosis.
Wrap edit
Wrap systems are solid, shape holding, microporous, non-tubular implants with diameters of less than 200 microns, which are not sutured or sealed. Wraps are the go to for current perivascular techniques but are only about average in each category, they are not difficult to implant, they have middling localization, and middling sustained release. They are the jack of all trades of the perivascular administration space, but that neutrality allows perivascular wraps to see broad possible uses.[4] Similar to cuff systems, the material makeup of the Wrap dictates the flexibility of the Wrap and therefore the amount of vasoconstriction necessary for the use case. In cases that require additional vasoconstrictions cuffs should be considered instead.[25]
Numerous wrap-based drug delivery systems have been proposed, some of the most representative examples include a PLGA based biodegradable wrap to locally deliver Sunitinib for treatment of hyperplasia,[10] a heparin eluting polyanhydride wrap which suppresses thrombosis,[26] and an EVAc + PEG wrap which elutes paclitaxel for hyperplasia risk management.[20]
Clinical uses edit
Methods of perivascular administration have been primarily proposed for the treatment of complications following vascular surgery, with most research being focused on the treatment of neointimal hyperplasia (IH).[4] Up to 40% of vein grafts occlude within 5 years of open vascular surgery,[27] thus bringing forth the need for localized treatment. Additionally, perivascular delivery has been explored for treating neointimal hyperplasia following arteriovenous fistula creation for hemodialysis[28].
Benefits and limitations edit
Perivascular drug delivery has several benefits over that of other means of drug delivery. This is especially true when taking into account the pathologies it treats. Given the localized nature of intimal hyperplasia, perivascular delivery is ideal for treatment. For example, one study found that localized delivery of mithramycin was more effective at attenuating neointimal hyperplasia than systemic administration[29]. Additionally, by utilizing the various system morphologies, perivascular delivery systems can also beneficially tailor the release profiles of a target drug allowing for immediate and/or controlled release. For example, combining hydrogel and PLGA microparticles allowed for both fast and controlled perivascular drug release of atorvastatin[6]. Certain systems, like gels, are also far superior than other perivascular delivery means as they can naturally degrade in the body and do not have to be removed at a later date[6]. Also, in the case of improving AVF outcomes systemic administration is limited by systemic toxicity issues, thus the localized delivery via perivascular systems are beneficial in this scenario[30]. One downside of perivascular administration is its inefficacy for systemic administration. That said, perivascular drug delivery systems are meant and designed to treat localized vascular conditions. Another downside of some perivascular systems, particularly gels, is that they offer no mechanical support to help treat initial hyperplasia. A literature review of perivascular meshes and grafts, however, found that constriction of the affected vessel helped reduce hyperplasia across various studies[4].
New developments and current research edit
Given its unique advantages, perivascular administration is being increasingly studied as an ideal means of treating various cardiovascular pathologies. One such pathology that has been the focus of recent research is intimal hyperplasia secondary to bypass grafting. Given differences in pharmacokinetics of various compounds used to treat intimal hyperplasia, various delivery systems are being developed for treatment. An atorvastatin delivery system composed of cross-linked hyaluronic acid hydrogel and PLGA microparticles has shown benefit in mice models for treating intimal hyperplasia with no observed toxic effects.[6] The combined efforts of immediate and controlled released provided by the use of both microparticles and hydrogels was seen to be most beneficial for reducing proliferation.[6] A follow-up study was completed in pig models, however the results seen in mice models were not reproduced.[31] This lack of reproduction was hypothesized to stem from challenges involved in dose adjustment across models. For example, an increased use of microparticles was necessary to comply with dose scaling which affected the thickness of the gel, thus hindering uniform application. In May 2022, researchers worked on improving hyaluronic acid hydrogels with dopamine for perivascular use.[32] This system also utilized pH-induced crosslinking to further aid adhesion. Later that year, further research incorporated the use of dopamine into the previous hyaluronic acid/microparticle delivery system to improve vascular adhesion.[33] This addition proved worthwhile as it not only increased the adhesive properties of the formulation, but also showed no observable effect on atorvastatin delivery kinetics and was tolerated well in rats. Delivery of atorvastatin is not the only compound for which perivascular administration is being explored. Perivascular delivery of resolvin D1, a specialized pro-resolving mediator, has also shown promise at reducing intimal hyperplasia in rabbit models.[34] It was demonstrated that delivery of resolvin D1 resulted in lower white blood cell recruitment and proliferation overall compared to vehicle controls. This benefit was observed in both bilayer PLGA wraps and pluronic gel.
There are also various recent studies looking to improve arteriovenous fistula maturation via reduction of intimal hyperplasia. Several systems have been and are being evaluated to deliver compounds such as Sirolimus[35], Simvastatin[36], 1α,25-dihydroxyvitamin D3 (Calcitrol)[37], Vonapanitase[38], β-aminopropionitrile[39], and 4-amino-1,8-naphtalamide[40]. For Sirolimus administration, there is currently an ongoing phase 3 clinical trial under government ID: NCT05425056. This uses a drug-eluting collagen implant, similar to other studies[35][41]. For Simvastatin, a preclinical trial in mice revealed significant reduction in expression of various genes (Vegf-A, etc.) that aide in stenosis, thus leading to less intimal cell proliferation[36]. Two preclinical studies in murine and porcine models using 1α,25-dihydroxyvitamin D3 reported successful attenuation of hyperplasia[37][42]. Both reported notable decreases in expression of IER3 (immediate early response 3) known to contribute to the development of intimal hyperplasia[42]. Two clinical trials looking at Vonapanitase administration were largely unsuccessful[38][43]. One trial found that local perivascular delivery of β-aminopropionitrile to inhibit lysyl oxidase, known to contribute to vessel fibrosis, was beneficial at improving circulation through rat AVF models[39]. Lastly, one trial using light-triggered delivery of 4-amino-1,8-naphtalamide was successful[40].
As a leading field in vascular surgery, perivascular drug delivery systems (PDDS) are being developed and enhanced in order to address complications such as intimal hyperplasia following surgery. Various innovative approaches are presented in this review to mitigate the risk of neointimal formation, which results in vascular graft failures. The studies in this area provide different perspectives on the challenges and solutions. For sustained drug delivery, Chen et al. (2017) develop a novel hybrid system based on unimolecular micelles embedded within a thermosensitive hydrogel. A triblock copolymer hydrogel was combined with rapamycin-loaded micelles, which demonstrated significant effectiveness in reducing the area ratio between the intima and media over three months in rats[44]. Unimolecular micelles offer enhanced drug loading capabilities over traditional nanoparticles due to their stability and enhanced stability[44]. Specifically in open surgical settings where other types of drug-eluting devices might not be viable, this research signifies a significant improvement in the short-term efficacy of existing vascular intervention techniques. Another study used silk fibroin microneedle wraps to target antiproliferative drugs directly to vascular injury sites[45]. In addition to achieving biocompatibility and mechanical compliance, silk fibroin is derived from Bombyx mori silkworm cocoons. By minimizing endothelial damage, microneedles enable precise drug delivery, reducing the adverse effects of more invasive approaches that have often been used in IH treatment. A similar concept was also employed in earlier experiments conducted by the same team, but the meshes used were transfer-molded biodegradable materials[46]. Compared to previous perivascular devices that can cause constriction or excessive localized drug concentrations, this design conforms well to the vascular anatomy, reduces mechanical stress, and ensures controlled medication release. Further contributions to the field were made by Sanders et al. (2012), who introduced a biodegradable wrap capable of controlled drug delivery, which degrades inside the body, eliminating long-term foreign body problems[47]. It mitigates IH, minimizes systemic side effects and maintains localization of the treatment (a challenge in systemic pharmacological treatments) while minimizing IH. As a collective, these studies highlight the rapid advances in PDDS, with its emphasis on biocompatibility, localized and sustained delivery of drugs, and mechanical compatibility with the vascular system.
Clinical trials edit
Various clinical trials based on Perivascular administration techniques are in progress, significant examples include:
Biocompound graft edit
Complete revascularization is the end goal of vascular surgery, for most patients the use of a left internal thoracic artery (LITA) graft yields high survivability. However, for some high-risk patients or those without suitable saphenous veins LITA grafts are unable to be used. The Biocompound Graft technique uses a perivascular mesh to allow for grafting in a wider number of cases. Clinical trials on over 200 patients found that for the average patient LITA grafting had superior patient outcomes over the Biocompound graft but that the Biocompound graft showed a significant improvement in patient outcomes for patients who were unsuitable for LITA grafting.[48]
This grafting technique was approved for use in the EU in 1997 and is currently approved by the FDA for US use.[48]
Vascugel edit
An endothelial cell-based matrix-type perivascular drug delivery system which is to be implanted to reduce the risks of graft thrombosis following vascular surgery. Phase 2 clinical trials have been completed and demonstrated improved patient outcomes over placebo.[49]
Coll-R edit
A drug eluting collagen wrap which focuses on the local delivery of Sirolimus to the perivascular space to reduce the risk of restenosis following vascular surgery. Currently in phase 3 clinical trials in the US.[50]
History edit
In the late 1980s research on local drug delivery to affected graft locations created the first perivascular administration techniques.[51] One of the first perivascular techniques researched was an attempt at using a solid silicon shell to surround an injected gel so as to improve localization of drug delivery.[5] However, the shell was unable to breakdown in in vitro environments and the research never advanced to clinical trials. In the 1990s, research on perivascular drug delivery largely focused on the use of heparin, an anticoagulant, for treating hyperplasia[4]. In the late 90s, other compounds were explored. For example, perivascular delivery of nitric oxide was showed to decrease intimal proliferation in 1998.[52] Sadly, over time while there have been large amounts of research into techniques of perivascular administration, there are few clinically significant applications of the techniques.[4]
References edit
- ^ a b Barcena, Allan (25 August 2022). "Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use". Biomolecules. 12 (10): 1367. doi:10.3390/biom12101367. PMC 9599524. PMID 36291576.
- ^ Seedial, Stephen (May 2013). "Local drug delivery to prevent restenosis". Journal of Vascular Surgery. 57 (5): 1403–1414. doi:10.1016/j.jvs.2012.12.069. PMC 3635112. PMID 23601595.
- ^ Wiedemann, Dominik (April 2012). "Perivascular administration of drugs and genes as a means of reducing vein graft failure". Current Opinion in Pharmacology. 12 (2): 203–216. doi:10.1016/j.coph.2012.02.012. PMID 22445655 – via Elsevier Science Direct.
- ^ a b c d e f g h i j k l m Mylonaki, Ioanna; Allémann, Éric; Saucy, François; Haefliger, Jacques-Antoine; Delie, Florence; Jordan, Olivier (June 2016). "Perivascular medical devices and drug delivery systems: Making the right choices". Biomaterials. 128: 56–68. doi:10.1016/j.biomaterials.2017.02.028. ISSN 0142-9612. PMID 28288349 – via Elsevier Science Direct.
- ^ a b Jones, Nicholas S.; Glenn, Michael G.; Orloff, Lisa A.; Mayberg, Marc R. (1990-07-01). "Prevention of Microvascular Thrombosis With Controlled-Release Transmural Heparin". Archives of Otolaryngology–Head & Neck Surgery. 116 (7): 779–785. doi:10.1001/archotol.1990.01870070027004. ISSN 0886-4470. PMID 2363913.
- ^ a b c d e Mylonaki, Ioanna; Strano, Francesco; Deglise, Sebastien; Allémann, Eric; Alonso, Florian; Corpataux, Jean-Marc; Dubuis, Céline; Haefliger, Jacques-Antoine; Jordan, Olivier; Saucy, François; Delie, Florence (June 2016). "Perivascular sustained release of atorvastatin from a hydrogel-microparticle delivery system decreases intimal hyperplasia". Journal of Controlled Release. 232: 93–102. doi:10.1016/j.jconrel.2016.04.023. ISSN 0168-3659. PMID 27091698.
- ^ Terry, Christi M.; Li, Li; Li, Huan; Zhuplatov, Ilya; Blumenthal, Donald K.; Kim, Seong-Eun; Owen, Shawn C.; Kholmovski, Eugene G.; Fowers, Kirk D.; Rathi, Ramesh; Cheung, Alfred K. (June 2012). "In vivo evaluation of the delivery and efficacy of a sirolimus-laden polymer gel for inhibition of hyperplasia in a porcine model of arteriovenous hemodialysis graft stenosis". Journal of Controlled Release. 160 (3): 459–467. doi:10.1016/j.jconrel.2012.03.011. ISSN 0168-3659. PMC 3372620. PMID 22465391.
- ^ Taguchi, J.; Abe, J.; Okazaki, H.; Ochiai, M.; Ohno, M.; Takuwa, Y.; Kurokawa, K. (Oct 1993). "Angiotensin Converting Enzyme Inhibitors or DuP753 Prevent Neointimal Formation Following Balloon Injury with Single Topical or Multiple Systemic Application". Biochemical and Biophysical Research Communications. 196 (2): 969–974. doi:10.1006/bbrc.1993.2344. ISSN 0006-291X. PMID 8240375.
- ^ Jeong, Byeongmoon; Bae, You Han; Kim, Sung Wan (May 2000). "In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof". Journal of Biomedical Materials Research. 50 (2): 171–177. doi:10.1002/(SICI)1097-4636(200005)50:2<171::AID-JBM11>3.0.CO;2-F. ISSN 0021-9304. PMID 10679681.
- ^ a b c Sanders, William G.; Hogrebe, Paul C.; Grainger, David W.; Cheung, Alfred K.; Terry, Christi M. (2012-07-10). "A biodegradable perivascular wrap for controlled, local and directed drug delivery". Journal of Controlled Release. 161 (1): 81–89. doi:10.1016/j.jconrel.2012.04.029. ISSN 0168-3659. PMC 3378780. PMID 22561340.
- ^ Melnik, Tamara; Ben Ameur, Senda; Kanfar, Nasreddine; Vinet, Laurent; Delie, Florence; Jordan, Olivier (January 2022). "Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking". International Journal of Molecular Sciences. 23 (10): 5706. doi:10.3390/ijms23105706. ISSN 1422-0067. PMC 9146728. PMID 35628516.
- ^ Sadowitz, Benjamin; Seymour, Keri; Gahtan, Vivian; Maier, Kristopher G. (Apr 2012). "The Role of Hyaluronic Acid in Atherosclerosis and Intimal Hyperplasia". Journal of Surgical Research. 173 (2): e63–e72. doi:10.1016/j.jss.2011.09.025. ISSN 0022-4804. PMID 22104612.
- ^ Emery, Robert W.; Solien, Eric; Puskas, John D. (Mar 2015). "Implantation of the eSVS Mesh: Modification of Recommended Technique". Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery. 10 (2): 146–149. doi:10.1097/imi.0000000000000130. ISSN 1556-9845. PMID 25807171.
- ^ Singh, Charanpreet; Wang, Xungai (Aug 2015). "A new design concept for knitted external vein-graft support mesh". Journal of the Mechanical Behavior of Biomedical Materials. 48: 125–133. doi:10.1016/j.jmbbm.2015.04.001. ISSN 1751-6161. PMID 25916819.
- ^ a b Zilla, Peter; Moodley, Loven; Wolf, Michael F.; Bezuidenhout, Deon; Sirry, Mazin S.; Rafiee, Nasser; Lichtenberg, Wilhelm; Black, Melanie; Franz, Thomas (Nov 2011). "Knitted nitinol represents a new generation of constrictive external vein graft meshes". Journal of Vascular Surgery. 54 (5): 1439–1450. doi:10.1016/j.jvs.2011.05.023. ISSN 0741-5214. PMID 21802240.
- ^ a b Mehta, Dheeraj; George, Sarah J.; Jeremy, Jamie Y.; Izzat, M. Bashar; Southgate, Kay M.; Bryan, Alan J.; Newby, Andrew C.; Angelini, Gianni D. (Feb 1998). "External stenting reduces long-term medial and neointimal thickening and platelet derived growth factor expression in a pig model of arteriovenous bypass grafting". Nature Medicine. 4 (2): 235–239. doi:10.1038/nm0298-235. ISSN 1546-170X. PMID 9461200.
- ^ Zilla, Peter; Wolf, Michael; Rafiee, Nasser; Moodley, Loven; Bezuidenhout, Deon; Black, Melanie; Human, Paul; Franz, Thomas (June 2009). "Utilization of shape memory in external vein-graft meshes allows extreme diameter constriction for suppressing intimal hyperplasia: A non-human primate study". Journal of Vascular Surgery. 49 (6): 1532–1542. doi:10.1016/j.jvs.2009.01.068. ISSN 0741-5214. PMID 19497517.
- ^ Murphy, Gavin J.; Newby, Andrew C.; Jeremy, Jamie Y.; Baumbach, Andreas; Angelini, Gianni D. (2007-08-01). "A randomized trial of an external Dacron sheath for the prevention of vein graft disease: The Extent study". The Journal of Thoracic and Cardiovascular Surgery. 134 (2): 504–505. doi:10.1016/j.jtcvs.2007.01.092. ISSN 0022-5223. PMID 17662798.
- ^ Huynh, Tam T.T.; Davies, Mark G.; Trovato, Matthew J.; Svendsen, Einar; Hagen, Per-Otto (Feb 1999). "Alterations in wall tension and shear stress modulate tyrosine kinase signaling and wall remodeling in experimental vein grafts". Journal of Vascular Surgery. 29 (2): 334–344. doi:10.1016/s0741-5214(99)70386-1. ISSN 0741-5214. PMID 9950991.
- ^ a b c Signore, Pierre E.; Machan, Lindsay S.; Jackson, John K.; Burt, Helen; Bromley, Peter; Wilson, Janet. E.; McManus, Bruce M. (Jan 2001). "Complete Inhibition of Intimal Hyperplasia by Perivascular Delivery of Paclitaxel in Balloon-injured Rat Carotid Arteries". Journal of Vascular and Interventional Radiology. 12 (1): 79–88. doi:10.1016/s1051-0443(07)61408-0. ISSN 1051-0443. PMID 11200358.
- ^ Fishbein, Ilia; Brauner, Ron; Chorny, Michael; Gao, Jianchuan; Chen, Xing; Laks, Hillel; Golomb, Gershon (Dec 2001). "Local delivery of mithramycin restores vascular reactivity and inhibits neointimal formation in injured arteries and vascular grafts". Journal of Controlled Release. 77 (3): 167–181. doi:10.1016/s0168-3659(01)00472-2. ISSN 0168-3659. PMID 11733085.
- ^ Burnett, Kelly (12 July 2006). "Perivascular paclitaxel wraps block arteriovenous graft stenosis in a pig model". academic.oup.com. Retrieved 2024-03-22.
- ^ Filova, Elena; Parizek, Martin; Olsovska, Jana; Kamenik, Zdenek; Brynda, Eduard; Riedel, Tomas; Vandrovcova, Marta; Lisa, Vera; Machova, Ludka; Skalsky, Ivo; Szarszoi, Ondrej; Suchy, Tomas; Bacakova, Lucie (Feb 2011). "Perivascular sirolimus-delivery system". International Journal of Pharmaceutics. 404 (1–2): 94–101. doi:10.1016/j.ijpharm.2010.11.005. ISSN 0378-5173. PMID 21075185.
- ^ Brauner, Ron; Laks, Hillel; Drinkwater, Davis C.; Chaudhuri, Gautam; Shvarts, Oleg; Drake, Thomas; Bhuta, Sunita; Mishaly, David; Fishbein, Ilia; Golomb, Gershon (July 1997). "Controlled periadventitial administration of verapamil inhibits neointimal smooth muscle cell proliferation and ameliorates vasomotor abnormalities in experimental vein bypass grafts". The Journal of Thoracic and Cardiovascular Surgery. 114 (1): 53–63. doi:10.1016/s0022-5223(97)70117-x. ISSN 0022-5223. PMID 9240294.
- ^ Yu, Xiaohua; Takayama, Toshio; Goel, Shakti A.; Shi, Xudong; Zhou, Yifan; Kent, K. Craig; Murphy, William L.; Guo, Lian-Wang (Oct 2014). "A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia". Journal of Controlled Release. 191: 47–53. doi:10.1016/j.jconrel.2014.05.017. ISSN 0168-3659. PMC 4156896. PMID 24852098.
- ^ Orloff, Lisa A.; Glenn, Michael G.; Domb, Abraham J.; Esclamado, Ramon A. (May 1995). "Prevention of venous thrombosis in microvascular surgery by transmural release of heparin from a polyanhydride polymer". Surgery. 117 (5): 554–559. doi:10.1016/s0039-6060(05)80255-7. ISSN 0039-6060. PMID 7740427.
- ^ Owens, Christopher D.; Gasper, Warren J.; Rahman, Amreen S.; Conte, Michael S. (Jan 2015). "Vein graft failure". Journal of Vascular Surgery. 61 (1): 203–216. doi:10.1016/j.jvs.2013.08.019. ISSN 0741-5214. PMC 4391818. PMID 24095042.
- ^ Barcena, Allan John R.; Perez, Joy Vanessa D.; Liu, Olivia; Mu, Amy; Heralde, Francisco M.; Huang, Steven Y.; Melancon, Marites P. (2022-09-24). "Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use". Biomolecules. 12 (10): 1367. doi:10.3390/biom12101367. ISSN 2218-273X. PMC 9599524. PMID 36291576.
- ^ Fishbein, Ilia; Brauner, Ron; Chorny, Michael; Gao, Jianchuan; Chen, Xing; Laks, Hillel; Golomb, Gershon (December 2001). "Local delivery of mithramycin restores vascular reactivity and inhibits neointimal formation in injured arteries and vascular grafts". Journal of Controlled Release. 77 (3): 167–181. doi:10.1016/s0168-3659(01)00472-2. ISSN 0168-3659. PMID 11733085.
- ^ Barcena, Allan John R.; Perez, Joy Vanessa D.; Liu, Olivia; Mu, Amy; Heralde, Francisco M.; Huang, Steven Y.; Melancon, Marites P. (2022-09-24). "Localized Perivascular Therapeutic Approaches to Inhibit Venous Neointimal Hyperplasia in Arteriovenous Fistula Access for Hemodialysis Use". Biomolecules. 12 (10): 1367. doi:10.3390/biom12101367. ISSN 2218-273X. PMC 9599524. PMID 36291576.
- ^ Saucy, Francois; Mylonaki, Ioanna; Déglise, Sébastien; Corpataux, Jean-marc; Dubuis, Céline; Jordan, Olivier; Delie, Florence (December 2019). "Perivascular Administration of Atorvastatin Loaded in Microparticles and Hyaluronic Acid Gel to Prevent Intimal Hyperplasia in Venous Graft". European Journal of Vascular and Endovascular Surgery. 58 (6): e99–e100. doi:10.1016/j.ejvs.2019.06.635. ISSN 1078-5884.
- ^ Melnik, Tamara; Ben Ameur, Senda; Kanfar, Nasreddine; Vinet, Laurent; Delie, Florence; Jordan, Olivier (January 2022). "Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking". International Journal of Molecular Sciences. 23 (10): 5706. doi:10.3390/ijms23105706. ISSN 1422-0067. PMC 9146728. PMID 35628516.
- ^ Melnik, Tamara; Porcello, Alexandre; Saucy, François; Delie, Florence; Jordan, Olivier (December 2022). "Bioadhesive Perivascular Microparticle-Gel Drug Delivery System for Intimal Hyperplasia Prevention: In Vitro Evaluation and Preliminary Biocompatibility Assessment". Gels. 8 (12): 776. doi:10.3390/gels8120776. ISSN 2310-2861. PMC 9778534. PMID 36547300.
- ^ Wu, Bian; Werlin, Evan C.; Chen, Mian; Mottola, Giorgio; Chatterjee, Anuran; Lance, Kevin D.; Bernards, Daniel A.; Sansbury, Brian E.; Spite, Matthew; Desai, Tejal A.; Conte, Michael S. (December 2018). "Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rabbit vein graft model". Journal of Vascular Surgery. 68 (6): 188S–200S.e4. doi:10.1016/j.jvs.2018.05.206. ISSN 0741-5214. PMC 6252159. PMID 30064835.
- ^ a b Clair, Daniel; Moritz, Michael; Burgess, Jason; Illig, Karl; Moldovan, Stefan; Parden, Justin; Ross, John; Iyer, Sri (August 2019). "Arteriovenous Fistula Outcomes After Local Vascular Delivery of a Sirolimus Formulation". Journal of Vascular Surgery. 70 (2): e37–e38. doi:10.1016/j.jvs.2019.06.048. ISSN 0741-5214.
- ^ a b Zhao, Chenglei; Zuckerman, Sean T.; Cai, Chuanqi; Kilari, Sreenivasulu; Singh, Avishek; Simeon, Michael; von Recum, Horst A.; Korley, Julius N.; Misra, Sanjay (2020-12-15). "Periadventitial Delivery of Simvastatin-Loaded Microparticles Attenuate Venous Neointimal Hyperplasia Associated With Arteriovenous Fistula". Journal of the American Heart Association. 9 (24): e018418. doi:10.1161/JAHA.120.018418. ISSN 2047-9980. PMC 7955373. PMID 33283594.
- ^ a b Singh, Avishek K.; Cai, Chuanqi; Kilari, Sreenivasulu; Zhao, Chenglei; Simeon, Michael L.; Takahashi, Edwin; Edelman, Elazer R.; Kong, Hyunjoon (Joon); Macedo, Thanila; Singh, Ravinder J.; Urban, Matthew W.; Kumar, Rajiv; Misra, Sanjay (April 2021). "1α,25-Dihydroxyvitamin D3 Encapsulated in Nanoparticles Prevents Venous Neointimal Hyperplasia and Stenosis in Porcine Arteriovenous Fistulas". Journal of the American Society of Nephrology. 32 (4): 866–885. doi:10.1681/ASN.2020060832. ISSN 1046-6673. PMC 8017547. PMID 33627344.
- ^ a b Peden, Eric K; Lucas, John F; Browne, Barry J; Settle, Stephen M; Scavo, Vincent A; Bleyer, Anthony J; Ozaki, Charles Keith; Teruya, Theodore H; Wilson, Samuel E; Mishler, Rick E; Ferris, Brian L; Hendon, Kendra S; Moist, Louise; Dixon, Bradley S; Wong, Marco D (March 2022). "PATENCY-2 trial of vonapanitase to promote radiocephalic fistula use for hemodialysis and secondary patency". The Journal of Vascular Access. 23 (2): 265–274. doi:10.1177/1129729820985626. ISSN 1129-7298. PMID 33482699.
- ^ a b Hernandez, Diana R.; Applewhite, Brandon; Martinez, Laisel; Laurito, Tyler; Tabbara, Marwan; Rojas, Miguel G.; Wei, Yuntao; Selman, Guillermo; Knysheva, Marina; Velazquez, Omaida C.; Salman, Loay H.; Andreopoulos, Fotios M.; Shiu, Yan-Ting; Vazquez-Padron, Roberto I. (February 2021). "Inhibition of Lysyl Oxidase with β-aminopropionitrile Improves Venous Adaptation after Arteriovenous Fistula Creation". Kidney360. 2 (2): 270–278. doi:10.34067/KID.0005012020. ISSN 2641-7650. PMC 8315119. PMID 34322674.
- ^ a b Shiu, Yan-Ting; He, Yuxia; Tey, Jason C. S.; Knysheva, Marina; Anderson, Blake; Kauser, Katalin (2021). "Natural Vascular Scaffolding Treatment Promotes Outward Remodeling During Arteriovenous Fistula Development in Rats". Frontiers in Bioengineering and Biotechnology. 9. doi:10.3389/fbioe.2021.622617. ISSN 2296-4185. PMC 7928390. PMID 33681159.
- ^ Paulson, W. D.; Kipshidze, N.; Kipiani, K.; Beridze, N.; DeVita, M. V.; Shenoy, S.; Iyer, S. S. (2012-03-01). "Safety and efficacy of local periadventitial delivery of sirolimus for improving hemodialysis graft patency: first human experience with a sirolimus-eluting collagen membrane (Coll-R)". Nephrology Dialysis Transplantation. 27 (3): 1219–1224. doi:10.1093/ndt/gfr667. ISSN 0931-0509. PMID 22241793.
- ^ a b Brahmbhatt, Akshaar; NievesTorres, Evelyn; Yang, Binxia; Edwards, William D.; Chaudhury, Prabir Roy; Lee, Min Kyun; Kong, Hyunjoon; Mukhopadhyay, Debabrata; Kumar, Rajiv; Misra, Sanjay (2014-07-18). "The Role of Iex-1 in the Pathogenesis of Venous Neointimal Hyperplasia Associated with Hemodialysis Arteriovenous Fistula". PLOS ONE. 9 (7): e102542. Bibcode:2014PLoSO...9j2542B. doi:10.1371/journal.pone.0102542. ISSN 1932-6203. PMC 4103828. PMID 25036043.
- ^ Bleyer, Anthony J.; Scavo, Vincent A.; Wilson, Samuel E.; Browne, Barry J.; Ferris, Brian L.; Ozaki, C. Keith; Lee, Timmy; Peden, Eric K.; Dixon, Bradley S.; Mishler, Rick; O'Connor, Timothy P.; Kidd, Kendrah; Burke, Steven K. (February 2019). "A randomized trial of vonapanitase (PATENCY-1) to promote radiocephalic fistula patency and use for hemodialysis". Journal of Vascular Surgery. 69 (2): 507–515. doi:10.1016/j.jvs.2018.04.068. ISSN 0741-5214. PMID 30683197.
- ^ a b Chen, Guojun; Shi, Xudong; Wang, Bowen; Xie, Ruosen; Guo, Lian-Wang; Gong, Shaoqin; Kent, K. Craig (2017-07-10). "Unimolecular Micelle-Based Hybrid System for Perivascular Drug Delivery Produces Long-Term Efficacy for Neointima Attenuation in Rats". Biomacromolecules. 18 (7): 2205–2213. doi:10.1021/acs.biomac.7b00617. ISSN 1525-7797. PMC 5927366. PMID 28613846.
- ^ Lee, JiYong; Jang, Eui Hwa; Kim, Jae Ho; Park, SeungHyun; Kang, Yosup; Park, Sanghyun; Lee, KangJu; Kim, Jung-Hwan; Youn, Young-Nam; Ryu, WonHyoung (2021-12-10). "Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery". Journal of Controlled Release. 340: 125–135. doi:10.1016/j.jconrel.2021.10.024. ISSN 0168-3659. PMID 34688718.
- ^ Lee, JiYong; Kim, Dae-Hyun; Lee, Kang Ju; Seo, Il Ho; Park, Seung Hyun; Jang, Eui Hwa; Park, Youngjoo; Youn, Young-Nam; Ryu, WonHyoung (2017-12-28). "Transfer-molded wrappable microneedle meshes for perivascular drug delivery". Journal of Controlled Release. 268: 237–246. doi:10.1016/j.jconrel.2017.10.007. ISSN 0168-3659. PMID 29030224.
- ^ Sanders, William G.; Hogrebe, Paul C.; Grainger, David W.; Cheung, Alfred K.; Terry, Christi M. (2012-07-10). "A biodegradable perivascular wrap for controlled, local and directed drug delivery". Journal of Controlled Release. 161 (1): 81–89. doi:10.1016/j.jconrel.2012.04.029. ISSN 0168-3659. PMC 3378780. PMID 22561340.
- ^ a b Zurbrügg, Heinz Robert; Knollmann, Friedrich; Musci, Michele; Wied, Markus; Bauer, Matthias; Chavez, Tito; Krukenberg, Andreas; Hetzer, Roland (November 2000). "The biocompound method in coronary artery bypass operations: surgical technique and 3-year patency". The Annals of Thoracic Surgery. 70 (5): 1536–1540. doi:10.1016/s0003-4975(00)01997-4. ISSN 0003-4975. PMID 11093483.
- ^ Conte, Michael S.; Nugent, Helen M.; Gaccione, Peter; Guleria, Indira; Roy-Chaudhury, Prabir; Lawson, Jeffrey H. (December 2009). "Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: The V-HEALTH study". Journal of Vascular Surgery. 50 (6): 1359–1368.e1. doi:10.1016/j.jvs.2009.07.108. ISSN 0741-5214. PMID 19958986.
- ^ Vascular Therapies, Inc. (2021-07-21). A Phase 3, Randomized, Multicenter, Single-blind, Controlled Study Evaluating Arteriovenous Fistula Outcomes With and Without a Perivascular Sirolimus-Eluting Collagen Implant (Report). clinicaltrials.gov.
- ^ Okada, Tomohisa; Bark, Don H.; Mayberg, Marc R. (Dec 1989). "Localized Release of Perivascular Heparin Inhibits Intimal Proliferation after Endothelial Injury without Systemic Anticoagulation". Neurosurgery. 25 (6): 892–898. doi:10.1227/00006123-198912000-00007. ISSN 0148-396X. PMID 2601819.
- ^ Chaux, Aurelio; Ruan, Xin Min; Fishbein, Michael C.; Ouyang, Yi; Kaul, Sanjay; Pass, Jennifer A.; Matloff, Jack M. (March 1998). "Perivascular Delivery Of A Nitric Oxide Donor Inhibits Neointimal Hyperplasia In Vein Grafts Implanted In The Arterial Circulation". The Journal of Thoracic and Cardiovascular Surgery. 115 (3): 604–614. doi:10.1016/s0022-5223(98)70325-3. ISSN 0022-5223. PMID 9535448.