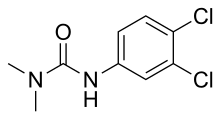
DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) is an algicide and herbicide of the aryl urea class that inhibits photosynthesis. It was introduced by Bayer in 1954 under the trade name of Diuron.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N′-(3,4-Dichlorophenyl)-N,N-dimethylurea | |
| Other names
3-(3,4-Dichlorophenyl)-1,1-dimethylurea, Karmex, Diuron, Direx
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.778 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077, 2767 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H10Cl2N2O | |
| Molar mass | 233.09 g·mol−1 |
| Appearance | white crystalline solid[1] |
| Density | 1.48 g/cm3 |
| Melting point | 158 °C (316 °F; 431 K) |
| Boiling point | 180 °C (356 °F; 453 K) |
| 42 mg/L | |
| Vapor pressure | 0.000000002 mmHg (20°C)[1] |
| Hazards | |
| GHS labelling: | |
  
| |
| Warning | |
| H302, H351, H373, H410 | |
| P201, P202, P260, P264, P270, P273, P281, P301+P312, P308+P313, P314, P330, P391, P405, P501 | |
| Flash point | noncombustible[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
TWA 10 mg/m3[1] |
IDLH (Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
History
editIn 1952, chemists at E. I. du Pont de Nemours and Company patented a series of aryl urea derivatives as herbicides. Several compounds covered by this patent were commercialized as herbicides: chlortoluron (3-chloro-4-methylphenyl) and DCMU, the (3,4-dichlorophenyl) example.[2][3] Subsequently, over thirty related urea analogs with the same mechanism of action reached the market worldwide.[4]
Synthesis
editAs described in the du Pont patent, the starting material is 3,4-dichloroaniline, which is treated with phosgene to form a isocyanate derivative. This is subsequently reacted with dimethylamine to give the final product.[2]
- Aryl-NH2 + COCl2 → Aryl-NCO
- Aryl-NCO + NH(CH3)2 → Aryl-NHCON(CH3)2
Mechanism of action
editDCMU is a very specific and sensitive inhibitor of photosynthesis. It blocks the QB plastoquinone binding site of photosystem II, disallowing the electron flow from photosystem II to plastoquinone.[5] This interrupts the photosynthetic electron transport chain in photosynthesis and thus reduces the ability of the plant to turn light energy into chemical energy (ATP and reductant potential).
DCMU only blocks electron flow from photosystem II, it has no effect on photosystem I or other reactions in photosynthesis, such as light absorption or carbon fixation in the Calvin cycle.[citation needed]
However, because it blocks electrons produced from water oxidation in PS II from entering the plastoquinone pool, "linear" photosynthesis is effectively shut down, as there are no available electrons to exit the photosynthetic electron flow cycle for reduction of NADP+ to NADPH. In fact, it was found that DCMU not only does not inhibit the cyclic photosynthetic pathway, but, under certain circumstances, actually stimulates it.[6][7]
Because of these effects, DCMU is often used to study energy flow in photosynthesis.
Toxicity
editDCMU (Diuron) has been characterized as a known/likely human carcinogen based on animal testing.[8][9]
References
edit- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0247". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b US patent 2655445, Todd C.W., "3-(Halophenyl)-1-methyl-1-(methyl or ethyl) ureas and herbicidal compositions and methods employing same", issued 1953-10-13, assigned to E. I. du Pont de Nemours & Co.
- ^ Liu, Jing (2010). "Phenylurea Herbicides". Hayes' Handbook of Pesticide Toxicology. pp. 1725–1731. doi:10.1016/B978-0-12-374367-1.00080-X. ISBN 9780123743671.
- ^ "Urea herbicides". alanwood.net. Retrieved 2021-03-26.
- ^ Metz, J; Pakrasi, H; Seibert, M; Arntzer, C (1986). "Evidence for a dual function of the herbicide-binding D1 protein in photosystem II". FEBS Letters. 205 (2): 269. doi:10.1016/0014-5793(86)80911-5. S2CID 84205263.
- ^ HUBER, S.C. EDWARDS, G.E. (1976), Studies on the Pathway of Cyclic Electron Flow in Mesophyll Chloroplasts of a C4 Plant, Biochimica et Biophysica Acta (BBA) - Bioenergetics, Volume 449, Issue 3, 6 December 1976, Pages 420-433, doi:10.1016/0005-2728(76)90153-5
- ^ Hosler, J. P.; Yocum, C. F. (April 1987). "Regulation of Cyclic Photophosphorylation during Ferredoxin-Mediated Electron Transport : Effect of DCMU and the NADPH/NADP Ratio". Plant Physiol. 83 (4): 965–9. doi:10.1104/pp.83.4.965. PMC 1056483. PMID 16665372.
- ^ "Diuron". National Center for Biotechnology Information. United States National Library of Medicine. Retrieved 9 November 2021.
- ^ Linda, Taylor; Esther, Rinde (1997-05-08). Carcinogenicity Peer Review of Diuron (PDF) (Memorandum). Washington, D.C.: United States Environmental Protection Agency. 20460.