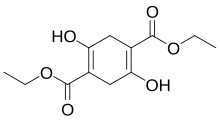
Diethylsuccinoylsuccinate is an organic compound with the formula [CH2C(OH)=C(CO2Et)]2 (Et = ethyl). A tetrasubstituted derivative of 1,4-cyclohexadiene, the compound is the enol tautomer of the corresponding cyclohexadione.[1] It is produced by base-induced condensation of diethyl succinate:[2]
| |
| Names | |
|---|---|
| Preferred IUPAC name
Diethyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate | |
| Other names
2,5-Dihydroxy-1,4-cyclohexadiene-1,4-dicarboxylic acid
1,4-Bis(ethoxycarbonyl)-2,5-dihydroxy-1,4-cyclohexadiene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H16O6 | |
| Molar mass | 256.254 g·mol−1 |
| Appearance | white solid |
| Density | 1.414 g/cm3 |
| Melting point | 125–126 °C (257–259 °F; 398–399 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
- 2 EtO2CCH2CH2CO2Et [CH2C(OH)=C(CO2Et)]2 + 2 EtOH
Diethylsuccinylsuccinate is valued as a precursor to the quinacridone pigments. For example, it reacts with two equiv of anilines to give the diamines [CH2C(N(H)Ar)=C(CO2Et)]2, which undergoes cyclization upon treatment with acid to give dihydroquinacridone.[3][4][5]
When heated in the presence of acid, diethylsuccinoylsuccinate converts to 1,4-cyclohexanedione via hydrolysis of the esters followed by decarboxylation.[2]
References edit
- ^ Mez, Hans-Christian; Rihs, Gret (1973). "Chemistry of Succinylsuccinic Acid Derivatives. Part II. The crystal and molecular structure of diethyl succinylsuccinate". Helvetica Chimica Acta. 56 (8): 2766–2772. doi:10.1002/hlca.19730560812.
- ^ a b Nielsen, Arnold T.; Carpenter, Wayne R. (1965). "1,4-Cyclohexanedione". Organic Syntheses. 45: 25. doi:10.15227/orgsyn.045.0025.
- ^ Hunger, K.; Herbst, W. (2012). "Pigments, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_371. ISBN 978-3527306732.(subscription required)
- ^ Smith, J. Anthony; West, Richard M.; Allen, Malcolm (2004). "Acridones and Quinacridones: Novel Fluorophores for Fluorescence Lifetime Studies". Journal of Fluorescence. 14 (2): 151–171. doi:10.1023/B:JOFL.0000016287.56322.eb.
- ^ Labana, S. S.; Labana, L. L. (1967). "Quinacridones". Chemical Reviews. 67: 1–18. doi:10.1021/cr60245a001.