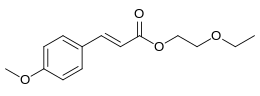
Cinoxate is an organic compound used as an ingredient in some types of sunscreens. It is an ester formed from methoxycinnamic acid and 2-ethoxyethanol. It is a slightly yellow viscous liquid that is insoluble in water, but miscible with alcohols, esters, and vegetable oils.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethoxyethyl (2E)-3-(4-methoxyphenyl)prop-2-enoate | |
| Other names
2-Ethoxyethyl p-methoxycinnamate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.901 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H18O4 | |
| Molar mass | 250.294 g·mol−1 |
| Density | 1.102 g/cm3 |
| Melting point | −25 °C (−13 °F; 248 K) |
| Boiling point | 184 to 187 °C (363 to 369 °F; 457 to 460 K) at 2 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It was approved as UV filter in the USA by the FDA in 1961, but it is not commonly used in cosmetic formulations anymore.[2]
See also
edit- Amiloxate, another methoxycinnamate-based sunscreen
- Octyl methoxycinnamate, another methoxycinnamate-based sunscreen
References
edit- ^ Merck Index, 11th Edition, 2312.
- ^ Pantelic, Molly N.; Wong, Nikita; Kwa, Michael; Lim, Henry W. (24 February 2023). "Ultraviolet filters in the United States and European Union: A review of safety and implications for the future of US sunscreens". Journal of the American Academy of Dermatology. 88 (3): 632–646. doi:10.1016/j.jaad.2022.11.039. PMID 36442641.