Caesium peroxide or cesium peroxide is an inorganic compound of caesium and oxygen with the chemical formula Cs2O2. It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly.[1]
| |
| Names | |
|---|---|
| IUPAC name
Caesium peroxide
| |
| Identifiers | |
| Properties | |
| Cs2O2 | |
| Molar mass | 297.809 g·mol−1 |
| Appearance | Yellowish[1] |
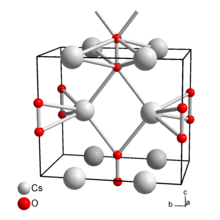
| Structure | |
| Orthorhombic[2] | |
| Related compounds | |
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
- 2 Cs + O2 → Cs2O2
It can also be formed by the thermal decomposition of caesium superoxide:[3]
- 2 CsO2 → Cs2O2 + O2
Upon heating until 650 °C, the compound will decompose to caesium monoxide and atomic oxygen:[4]
- Cs2O2 → Cs2O + [O]
Caesium peroxide shows a Raman vibration at 743 cm−1, due to the presence of the peroxide ions.[5] The compound is often used as a coating for photocathodes, due to its low work function.[6]
References
edit- ^ a b I. I. Volnov (2012). Peroxides, Superoxides, and Ozonides of Alkali and Alkaline Earth Metals. Springer. p. 45. ISBN 9781468482522.
- ^ Band, A.; Albu-Yaron, A.; Livneh, T.; Cohen, H.; Feldman, Y.; Shimon, L.; Popovitz-Biro, R.; Lyahovitskaya, V.; Tenne, R. (2004-07-27). "Characterization of Oxides of Cesium". The Journal of Physical Chemistry B. 108 (33). American Chemical Society (ACS): 12360–12367. doi:10.1021/jp036432o. ISSN 1520-6106.
- ^ Berardinelli, S. P.; Kraus, D. L. (1974-01-01). "Thermal decomposition of the higher oxides of cesium in the temperature range 320-500.deg". Inorganic Chemistry. 13 (1). American Chemical Society (ACS): 189–191. doi:10.1021/ic50131a037. ISSN 0020-1669.
- ^ Zefirov, Nikolaj (1995). Chimičeskaja ėnciklopedija : v pjati tomach (in Bosnian). Moskva: Izdat. p. 658. ISBN 5-85270-092-4. OCLC 258155382.
- ^ Livneh, Tsachi; Band, Alisa; Tenne, Reshef (2002). "Raman scattering from the peroxide ion in Cs2O2". Journal of Raman Spectroscopy. 33 (8). Wiley: 675–676. Bibcode:2002JRSp...33..675L. doi:10.1002/jrs.900. ISSN 0377-0486.
- ^ Sun, Yun; Liu, Zhi; Pianetta, Piero; Lee, Dong-Ick (2007). "Formation of cesium peroxide and cesium superoxide on InP photocathodes activated by cesium and oxygen". Journal of Applied Physics. 102 (7). AIP Publishing: 074908–074908–6. Bibcode:2007JAP...102g4908S. doi:10.1063/1.2786882. ISSN 0021-8979.
External links
edit