Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.[7][3]
| |

| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.704 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 1564 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaCl2 | |
| Molar mass | 208.23 g/mol (anhydrous) 244.26 g/mol (dihydrate) |
| Appearance | White powder, or colourless or white crystals (anhydrous) Colourless rhomboidal crystals (dihydrate)[2][3] |
| Odor | Odourless |
| Density | 3.856 g/cm3 (anhydrous) 3.0979 g/cm3 (dihydrate) |
| Melting point | 962 °C (1,764 °F; 1,235 K) (960 °C, dihydrate) |
| Boiling point | 1,560 °C (2,840 °F; 1,830 K) |
| |
| Solubility | Soluble in methanol, insoluble ethyl acetate, slightly soluble in hydrochloric acid and nitric acid, very slightly soluble in ethanol.[4][3] The dihydrate of barium chloride is soluble in methanol, almost insoluble in ethanol, acetone and ethyl acetate.[3] |
| −72.6·10−6 cm3/mol | |
| Structure | |
| PbCl2-type orthorhombic (anhydrous) monoclinic (dihydrate) | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
123.9 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−858.56 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly toxic, corrosive |
| GHS labelling: | |

| |
| Danger | |
| H301, H302, H332 | |
| P261, P264, P270, P271, P301+P310, P304+P312, P304+P340, P312, P321, P330, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
78 mg/kg (rat, oral) 50 mg/kg (guinea pig, oral)[6] |
LDLo (lowest published)
|
112 mg/kg (as Ba) (rabbit, oral) 59 mg/kg (as Ba) (dog, oral) 46 mg/kg (as Ba) (mouse, oral)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3[5] |
REL (Recommended)
|
TWA 0.5 mg/m3[5] |
IDLH (Immediate danger)
|
50 mg/m3[5] |
| Safety data sheet (SDS) | NIH BaCl |
| Related compounds | |
Other anions
|
|
Other cations
|
|
| Supplementary data page | |
| Barium chloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editOn an industrial scale, barium chloride is prepared via a two step process from barite (barium sulfate).[8] The first step requires high temperatures.
- BaSO4 + 4 C → BaS + 4 CO
The second step requires reaction between barium sulfide and hydrogen chloride:
- BaS + 2 HCl → BaCl2 + H2S
or between barium sulfide and calcium chloride:
- BaS + CaCl2 → CaS + BaCl2[2]
In place of HCl, chlorine can be used.[7] Barium chloride is extracted out from the mixture with water. From water solutions of barium chloride, its dihydrate (BaCl2·2H2O) can be crystallized as colorless crystals.[2]
Barium chloride can in principle be prepared by the reaction between barium hydroxide or barium carbonate with hydrogen chloride. These basic salts react with hydrochloric acid to give hydrated barium chloride.
- Ba(OH)2 + 2 HCl → BaCl2 + 2 H2O
- BaCO3 + 2 HCl → BaCl2 + H2O + CO2
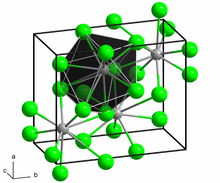
Structure and properties
editBaCl2 crystallizes in two forms (polymorphs). At room temperature, the compound is stable in the orthorhombic cotunnite (PbCl2) structure, whereas the cubic fluorite structure (CaF2) is stable between 925 and 963 °C.[9] Both polymorphs accommodate the preference of the large Ba2+ ion for coordination numbers greater than six.[10] The coordination of Ba2+ is 8 in the fluorite structure[11] and 9 in the cotunnite structure.[12] When cotunnite-structure BaCl2 is subjected to pressures of 7–10 GPa, it transforms to a third structure, a monoclinic post-cotunnite phase. The coordination number of Ba2+ increases from 9 to 10.[13]
In aqueous solution BaCl2 behaves as a simple salt; in water it is a 1:2 electrolyte[clarification needed] and the solution exhibits a neutral pH. Its solutions react with sulfate ion to produce a thick white solid precipitate of barium sulfate.
- BaCl2 + Na2SO4 → 2 NaCl + BaSO4
This precipitation reaction is used in chlor-alkali plants to control the sulfate concentration in the feed brine for electrolysis.
Oxalate effects a similar reaction:
When it is mixed with sodium hydroxide, it gives barium hydroxide, which is moderately soluble in water.
- BaCl2 + 2 NaOH → 2 NaCl + Ba(OH)2
BaCl2·2H2O is stable in the air at room temperature, but loses one water of crystallization above 55 °C (131 °F), becoming BaCl2·H2O, and becomes anhydrous above 121 °C (250 °F).[2] BaCl2·H2O may be formed by shaking the dihydrate with methanol.[3]
BaCl2 readily forms eutectics with alkali metal chlorides.[3]
Uses
editAlthough inexpensive, barium chloride finds limited applications in the laboratory and industry.
Its main laboratory use is as a reagent for the gravimetric determination of sulfates. The sulfate compound being analyzed is dissolved in water and hydrochloric acid is added. When barium chloride solution is added, the sulfate present precipitates as barium sulfate, which is then filtered through ashless filter paper. The paper is burned off in a muffle furnace, the resulting barium sulfate is weighed, and the purity of the sulfate compound is thus calculated.
In industry, barium chloride is mainly used in the purification of brine solution in caustic chlorine plants and also in the manufacture of heat treatment salts, case hardening of steel.[7] It is also used to make red pigments such as Lithol red and Red Lake C. Its toxicity limits its applicability.[citation needed]
Toxicity
editBarium chloride, along with other water-soluble barium salts, is highly toxic.[14] It irritates eyes and skin, causing redness and pain. It damages kidneys. Fatal dose of barium chloride for a human has been reported to be about 0.8-0.9 g. Systemic effects of acute barium chloride toxicity include abdominal pain, diarrhea, nausea, vomiting, cardiac arrhythmia, muscular paralysis, and death. The Ba2+ ions compete with the K+ ions, causing the muscle fibers to be electrically unexcitable, thus causing weakness and paralysis of the body.[3] Sodium sulfate and magnesium sulfate are potential antidotes because they form barium sulfate BaSO4, which is relatively non-toxic because of its insolubility in water.
Barium chloride is not classified as a human carcinogen.[3]
References
edit- ^ Chemical Recreations: A Series of Amusing and Instructive Experiments, which May be Performed with Ease, Safety, Success, and Economy ; to which is Added, the Romance of Chemistry : An Inquiry into the Fallacies of the Prevailing Theory of Chemistry : With a New Theory and a New Nomenclature. R. Griffin & Company. 1834.
- ^ a b c d "Barium Chloride - an overview | ScienceDirect Topics".
- ^ a b c d e f g h "Barium chloride".
- ^ Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0045". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Barium (soluble compounds, as Ba)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Kresse, Robert; Baudis, Ulrich; Jäger, Paul; Riechers, H. Hermann; Wagner, Heinz; Winkler, Jocher; Wolf, Hans Uwe (2007). "Barium and Barium Compounds". In Ullman, Franz (ed.). Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a03_325.pub2. ISBN 978-3527306732.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Edgar, A.; Zimmermann, J.; von Seggern, H.; Varoy, C. R. (2010-04-15). "Lanthanum-stabilized europium-doped cubic barium chloride: An efficient x-ray phosphor". Journal of Applied Physics. 107 (8). AIP Publishing: 083516–083516–7. Bibcode:2010JAP...107h3516E. doi:10.1063/1.3369162. ISSN 0021-8979.
- ^ Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Haase, A.; Brauer, G. (1978). "Hydratstufen und Kristallstrukturen von Bariumchlorid". Z. anorg. allg. Chem. 441: 181–195. doi:10.1002/zaac.19784410120.
- ^ Brackett, E. B.; Brackett, T. E.; Sass, R. L. (1963). "The Crystal Structures of Barium Chloride, Barium Bromide, and Barium Iodide". J. Phys. Chem. 67 (10): 2132. doi:10.1021/j100804a038.
- ^ Léger, J. M.; Haines, J.; Atouf, A. (1995). "The Post-Cotunnite Phase in BaCl2, BaBr2 and BaI2 under High Pressure". J. Appl. Crystallogr. 28 (4): 416. Bibcode:1995JApCr..28..416L. doi:10.1107/S0021889895001580.
- ^ The Merck Index, 7th edition, Merck & Co., Rahway, New Jersey, 1960.
