Amixetrine (INN) (brand name Somagest; developmental code name CERM-898) is a drug that was formerly marketed in France but is now no longer sold.[1][2] According to various sources it has been said to be an anti-inflammatory, antidepressant, antispasmodic, anticholinergic, antihistamine, and antiserotonergic, but its definitive indications and pharmacology are unclear.[1][2] The drug was first synthesized in 1969 and was introduced in France in 1972.[1][2]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
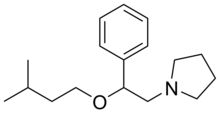
| Formula | C17H27NO |
| Molar mass | 261.409 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Synthesis
editThe treatment of isoamyl alcohol (1) with styrene (2) at -10°C with dropwise addition of tert-Butyl Hypobromite [1611-82-1] gives (2-bromo-1-(isopentyloxy)ethyl)benzene [5452-50-6] (3). Displacement of the halogen leaving group by pyrrolidine completes the synthesis of amixetrine (4).
References
edit- ^ a b c Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 80–. ISBN 978-1-4757-2085-3.
- ^ a b c William Andrew Publishing (22 October 2013). "Amixetrine Hydrochloride". Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 285–. ISBN 978-0-8155-1856-3.
- ^ GB 1253818 idem Mauvernay Roland Yves, Norbert Busch, DE1811767A1 (1969 to Ct Europ De Rech S Mauvernay).
- ^ Finkelstein, B. L., Hu, T. (15 March 2009). "Encyclopedia of Reagents for Organic Synthesis". In John Wiley & Sons, Ltd (eds.). tert -Butyl Hypobromite. John Wiley & Sons, Ltd. pp. rb387.pub2. doi:10.1002/047084289X.rb387.pub2.