1,3-Dithietane is a dithietane. It is a colorless, crystalline, unpleasant-smelling solid. It was first prepared in 1976 by the reaction of bis(chloromethyl) sulfoxide with sodium sulfide to give 1,3-dithietane 1-oxide, followed by THF-borane reduction.[1][2]
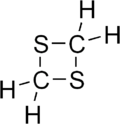
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Dithietane | |
| Systematic IUPAC name
1,3-Dithiacyclobutane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H4S2 | |
| Molar mass | 92.18 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Examples of compounds bearing this functional group include the antibiotic Cefotetan and the pesticide Fosthietan.
References
edit- ^ Block, E; Corey, ER; Penn, RE; Renken, TL; Sherwin, PF (1976). "1,3-Dithietane". J. Am. Chem. Soc. 98 (18): 5715–5717. doi:10.1021/ja00434a061.
- ^ Block, E; Corey, ER; Penn, RE; Renken, TL; Sherwin, PF; Bock, H; Hirabayashi, T; Mohmand, S; Solouki, B (1982). "Synthesis and Thermal Decomposition of 1,3-Dithietane and its S-Oxides". J. Am. Chem. Soc. 104 (11): 3119–3130. doi:10.1021/ja00375a030.