Trifluoromethylsulfur pentafluoride
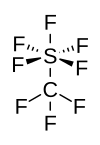
Trifluoromethylsulfur pentafluoride, CF3SF5, is a rarely used industrial greenhouse gas.[1] It was first identified in the atmosphere in 2000.[2] Trifluoromethylsulfur pentafluoride is considered to be one of the several "super-greenhouse gases".
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentafluoro(trifluoromethyl)-λ6-sulfane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.196.530 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CF8S | |
| Molar mass | 196.06 g·mol−1 |
| Melting point | −87 °C (−125 °F; 186 K) |
| Boiling point | −20.4 °C (−4.7 °F; 252.8 K) |
| Hazards | |
| GHS labelling: | |
  
| |
| H315, H319, H335, H336 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P403+P233, P405, P410+P403, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Properties
editThe chemistry of this compound is similar to that of sulfur hexafluoride (SF6).[3]
As a greenhouse gas
editOn a per molecule basis, it is considered to be the most potent greenhouse gas present in Earth's atmosphere, having a global warming potential of about 18,000 times that of carbon dioxide.[4] The chemical is predicted to have a lifetime of 800 years in the atmosphere.[5] However, the current concentration of trifluoromethylsulfur pentafluoride remains at a level that is unlikely to measurably contribute to global warming.[4] The presence of the gas in the atmosphere is attributed to anthropogenic sources, possibly a by-product of the manufacture of fluorochemicals, originating from reactions of SF6 with fluoropolymers used in electronic devices and in microchips, or the formation can be associated with high voltage equipment created from SF6 (a breakdown product of high voltage equipment) reacting with CF3 to form the CF3SF5 molecule.[4]
References
edit- ^ Tuckett, Richard P. (2006). "Chapter 3: Trifluoromethyl Sulphur Pentafluoride, SF5CF3: Atmospheric Chemistry and Its Environmental Importance via the Greenhouse Effect". In Tressaud, Alain (ed.). Fluorine and the Environment Chemistry, Emissions, & Lithosphere (Advances in Fluorine Science series) (PDF). Vol. 1. Elsevier. pp. 89–129. doi:10.1016/S1872-0358(06)01003-7. ISBN 0-444-52811-3.
- ^ Sturges, W.T.; T. J. Wallington; M. D. Hurley; K. P. Shine; K. Sihra; A. Engel; D. E. Oram; S. A. Penkett; R. Mulvaney; C.A.M. Brenninkmeijer (28 July 2000). "A Potent Greenhouse Gas Identified in the Atmosphere: SF5CF3". Science. 289 (5479): 611–613. Bibcode:2000Sci...289..611S. doi:10.1126/science.289.5479.611. PMID 10915622.
- ^ Silvey, Gene A.; Cady, George H. (1950). "Trifluoromethylsulfur Pentafluoride". J. Am. Chem. Soc. 72 (8): 3624–3626. doi:10.1021/ja01164a084.
- ^ a b c Suen, Martin (2008). "Trifluoromethyl Sulfur Pentafluoride (CF3SF5): A Review of the Recently Discovered Super-Greenhouse Gas in the Atmosphere". The Open Atmospheric Science Journal. 2 (1): 56–60. Bibcode:2008OASJ....2...56S. doi:10.2174/1874282300802010056.
- ^ Tsai, Wen-Tien (2007). "The prediction of environmental fate for trifluoromethyl sulfur pentafluoride (CF3SF5), a potent greenhouse gas". Journal of Hazardous Materials. 149 (3): 747–751. doi:10.1016/j.jhazmat.2007.08.035. PMID 17884286.
