Titanium tetrabromide is the chemical compound with the formula TiBr4. It is the most volatile transition metal bromide. The properties of TiBr4 are an average of TiCl4 and TiI4. Some key properties of these four-coordinated Ti(IV) species are their high Lewis acidity and their high solubility in nonpolar organic solvents. TiBr4 is diamagnetic, reflecting the d0 configuration of the metal centre.[2]
| |
| |
| Names | |
|---|---|
| IUPAC name
Titanium(IV) bromide
| |
| Other names
Titanium tetrabromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.029.259 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TiBr4 | |
| Molar mass | 367.483 g/mol |
| Appearance | brown crystals hygroscopic |
| Density | 3.25 g/cm3 |
| Melting point | 39 °C (102 °F; 312 K) |
| Boiling point | 230 °C (446 °F; 503 K) |
| hydrolyses | |
| Solubility in other solvents | chlorocarbons, benzene |
| Structure | |
| cubic, Pa3, Z = 8 | |
| Tetrahedral | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
corrosive |
| GHS labelling:[1] | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P363, P405 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Titanium(IV) chloride Titanium(IV) fluoride Titanium(IV) iodide |
Related compounds
|
Titanium(III) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation and structure
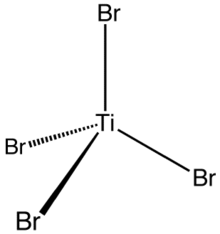
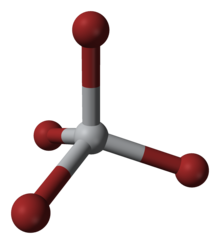
editThis four-coordinated complex adopts a tetrahedral geometry. It can be prepared via several methods: (i) from the elements, (ii) via the reaction of TiO2 with carbon and bromine (see Kroll process), and (iii) by treatment of TiCl4 with HBr.
Reactions
editTitanium tetrabromide forms adducts such as TiBr4(THF)2 and [TiBr5]−.[3] With bulky donor ligands, such as 2-methylpyridine (2-Mepy), five-coordinated adducts form. TiBr4(2-MePy) is trigonal bipyramidal with the pyridine in the equatorial plane.[4]
TiBr4 has been used as a Lewis-acid catalyst in organic synthesis.[5]
The tetrabromide and tetrachlorides of titanium react to give a statistical mixture of the mixed tetrahalides, TiBr4−xClx (x = 0-4). The mechanism of this redistribution reaction is uncertain. One proposed pathway invokes the intermediacy of dimers.[6]
Safety
editTiBr4 hydrolyzes rapidly, potentially dangerously, to release hydrogen bromide, otherwise known as hydrobromic acid.
References
edit- ^ "Titanium tetrabromide". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Colin S. Creaser & J. Alan Creighton (1975). "Pentachloro- and pentabromo-titanate(IV) ions". J. Chem. Soc., Dalton Trans. (14): 1402–1405. doi:10.1039/DT9750001402.
- ^ Hensen, K.; Lemke, A.; Bolte, M. (2000). "Tetrabromo(2-methylpyridine-N)-titanate(IV)". Acta Crystallographica. C56 (12): e565–e566. Bibcode:2000AcCrC..56E.565H. doi:10.1107/S0108270100015407.
- ^ B. Patterson, S. Marumoto & S. D. Rychnovsky (2003). "Titanium(IV)-Promoted Mukaiyama Aldol-Prins Cyclizations". Org. Lett. 5 (17): 3163–3166. doi:10.1021/ol035303n. PMID 12917007.
- ^ S. P. Webb & M. S. Gordon (1999). "Intermolecular Self-Interactions of the Titanium Tetrahalides TiX4 (X = F, Cl, Br)". J. Am. Chem. Soc. 121 (11): 2552–2560. doi:10.1021/ja983339i.
