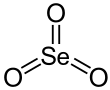
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.[4]
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.033.972 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SeO3 | |||
| Molar mass | 126.96 g/mol | ||
| Appearance | white hygroscopic crystals | ||
| Density | 3.44 g/cm3 | ||
| Melting point | 118.35 °C (245.03 °F; 391.50 K) | ||
| Boiling point | sublimes | ||
| very soluble | |||
| Structure | |||
| tetragonal | |||
| Hazards | |||
| GHS labelling:[3] | |||
 
| |||
| Danger | |||
| H301, H331, H373, H410 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
7 mg/kg (rat, oral) 7.08 mg/kg (mouse, oral) 5.06 mg/kg (guinea pig, oral) 2.25 mg/kg (rabbit, oral) 13 mg/kg (horse, oral)[2] | ||
LC50 (median concentration)
|
13 mg/kg (pig, oral) 9.9 mg/kg (cow, oral) 3.3 mg/kg (goat, oral) 3.3 mg/kg (sheep, oral)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Preparation
editSelenium trioxide is difficult to prepare because it is unstable with respect to the dioxide:
- 2 SeO3 → 2 SeO2 + O2
It has been generated in a number of ways despite the fact that the dioxide does not combust under normal conditions.[4] One method entails dehydration of anhydrous selenic acid with phosphorus pentoxide at 150–160 °C. Another method is the reaction of liquid sulfur trioxide with potassium selenate.
- SO3 + K2SeO4 → K2SO4 + SeO3
Reactions
editIn its chemistry SeO3 generally resembles sulfur trioxide, SO3, rather than tellurium trioxide, TeO3.[4] The substance reacts explosively with oxidizable organic compounds.[5]
At 120 °C SeO3 reacts with selenium dioxide to form the Se(VI)-Se(IV) compound diselenium pentaoxide:[6]
- SeO3 + SeO2 → Se2O5
It reacts with selenium tetrafluoride to form selenoyl fluoride, the selenium analogue of sulfuryl fluoride
- 2SeO3 + SeF4 → 2SeO2F2 + SeO2
As with SO3 adducts are formed with Lewis bases such as pyridine, dioxane and ether.[4]
With lithium oxide and sodium oxide it reacts to form salts of SeVIO54− and SeVIO66−:[7] With Li2O, it gives Li4SeO5, containing the trigonal pyramidal anion SeVIO54− with equatorial bonds, 170.6–171.9 pm; and longer axial Se−O bonds of 179.5 pm. With Na2O it gives Na4SeO5, containing the square pyramidal SeVIO54−, with Se−O bond lengths ranging from range 172.9 → 181.5 pm, and Na12(SeO4)3(SeO6), containing octahedral SeVIO66−. SeVIO66− is the conjugate base of the unknown orthoselenic acid (Se(OH)6).
Structure
editIn the solid phase SeO3 consists of cyclic tetramers, with an 8 membered (Se−O)4 ring. Selenium atoms are 4-coordinate, bond lengths being Se−O bridging are 175 pm and 181 pm, non-bridging 156 and 154 pm.[7]
SeO3 in the gas phase consists of tetramers and monomeric SeO3 which is trigonal planar with an Se−O bond length of 168.78 pm.[8]
References
edit- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–81. ISBN 0-8493-0594-2.
- ^ a b "Selenium compounds (as Se)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "C&L Inventory". echa.europa.eu. Retrieved 16 December 2021.
- ^ a b c d Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- ^ Schmidt, Bornmann & Wilhelm 1963.
- ^ Z. Žák "Crystal structure of diselenium pentoxide Se2O5" Zeitschrift für anorganische und allgemeine Chemie 1980, volume 460, pp. 81–85. doi:10.1002/zaac.19804600108
- ^ a b Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, Francesco A. Devillanova, Royal Society of Chemistry, 2007, ISBN 9780854043668
- ^ Brassington, N. J.; Edwards, H. G. M.; Long, D. A.; Skinner, M. (1978). "The pure rotational Raman spectrum of SeO3". Journal of Raman Spectroscopy. 7 (3): 158–160. Bibcode:1978JRSp....7..158B. doi:10.1002/jrs.1250070310. ISSN 0377-0486.
Further reading
edit- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Schmidt, Prof. Dr. Max; Bornmann, Dr. P.; Wilhelm, Dr. Irmgard (1963-10-02). "The Chemistry of Selenium Trioxide". Angewandte Chemie International Edition in English. 2 (11): 691–692. doi:10.1002/anie.196306913.