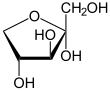
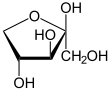
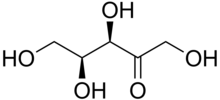
Xylulose is a ketopentose, a monosaccharide containing five carbon atoms, and including a ketone functional group. It has the chemical formula C5H10O5. In nature, it occurs in both the L- and D-enantiomers.[3] 1-Deoxyxylulose is a precursor to terpenes via the DOXP pathway.[4]
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
L-threo-Pent-2-ulose
| |||
| Systematic IUPAC name
(3R,4S)-1,3,4,5-Tetrahydroxypentan-2-one | |||
| Other names
threo-Pentulose
threo-2-PentuloseL-Xylulose | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H10O5 | |||
| Molar mass | 150.130 g·mol−1 | ||
| Appearance | colorless syrup | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Pathology
editL-Xylulose accumulates in the urine in patients with pentosuria, due to a deficiency in L-xylulose reductase. Since L-xylulose is a reducing sugar like D-glucose, pentosuria patients have been wrongly diagnosed in the past to be diabetic.
References
edit- ^ Data is for L-xylulose.
- ^ Merck Index, 11th Edition, 9996.
- ^ Winkelhausen, Eleonora; Kuzmanova, Slobodanka (1998). "Microbial conversion of d-xylose to xylitol". Journal of Fermentation and Bioengineering. 86: 1–14. doi:10.1016/S0922-338X(98)80026-3.
- ^ Rohdich, F.; Bacher, A.; Eisenreich, W. (2005). "Isoprenoid biosynthetic pathways as anti-infective drug targets". Biochemical Society Transactions. 33 (4): 785–791. doi:10.1042/BST0330785. PMID 16042599.