Large for gestational age (LGA) is a term used to describe infants that are born with an abnormally high weight, specifically in the 90th percentile or above, compared to other babies of the same developmental age.[1][2][3] Macrosomia is a similar term that describes excessive birth weight, but refers to an absolute measurement, regardless of gestational age.[4] Typically the threshold for diagnosing macrosomia is a body weight between 4,000 and 4,500 grams (8 lb 13 oz and 9 lb 15 oz), or more, measured at birth, but there are difficulties reaching a universal agreement of this definition.[4]
| Large for gestational age | |
|---|---|
| Other names | Macrosomia |
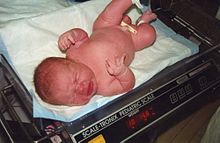 | |
| LGA: A healthy 11-pound (5.0 kg) newborn child, delivered vaginally without complications (41 weeks; fourth child; no gestational diabetes) | |
| Specialty | Obstetrics, pediatrics |
Evaluating an infant for macrosomia or LGA can help identify risks associated with their birth, including labor complications of both the parent and the child, potential long-term health complications of the child, and infant mortality.[5]
Signs and symptoms
editFetal macrosomia and LGA often do not present with noticeable patient symptoms. Important signs include large fundal height (uterus size) and excessive amniotic fluid (polyhydramnios).[6] Fundal height can be measured from the top of the uterus to the pubic bone and indicates that the newborn is likely large in volume. Excessive amniotic fluid indicates that the fetus’ urine output is larger than expected, indicating a larger baby than normal; some symptoms of excessive amniotic fluid include
- shortness of breath
- swelling of lower extremities & abdominal wall
- uterine discomfort or contractions
- fetal malposition, such as breech presentation.[6]
Complications
editLGA or macrosomic births can lead to complications for both the mother and the infant.[7]
Infant complications
editCommon risks in LGA babies include shoulder dystocia,[5] hypoglycemia,[5] brachial plexus injuries,[8] metatarsus adductus, hip subluxation[9] and talipes calcaneovalgus, due to intrauterine deformation.[9]
Shoulder dystocia occurs when the infant's shoulder becomes impacted on the mother's pubic symphysis during birth.[10] Newborns with shoulder dystocia are at risk of temporary or permanent nerve damage to the baby's arm, or other injuries such as fracture.[11] Both increased birth weight and diabetes in the gestational parent are independent risk factors seen to increase risk of shoulder dystocia.[11] In non-diabetic women, shoulder dystocia happens 0.65% of the time in babies that weigh less than 8 pounds 13 ounces (4,000 g), 6.7% of the time in babies that weigh 8 pounds (3,600 g) to 9 pounds 15 ounces (4,500 g), and 14.5% of the time in babies that weigh more than 9 pounds 15 ounces (4,500 g).[12] In diabetic women, shoulder dystocia happens 2.2% of the time in babies that weigh less than 8 pounds 13 ounces (4,000 g), 13.9% of the time in babies that weigh 8 pounds (3,600 g) to 9 pounds 15 ounces (4,500 g), and 52.5% of the time in babies that weigh more than 9 pounds 15 ounces (4,500 g).[12] Although larger babies are at higher risk for shoulder dystocia, most cases of shoulder dystocia happen in smaller babies because there are many more small and normal-size babies being born than large babies.[13]
LGA babies are at higher risk of hypoglycemia in the neonatal period, independent of whether the mother has diabetes.[14] Hypoglycemia, as well as hyperbilirubinemia and polycythemia, occurs as a result of hyperinsulinemia in the fetus.[15]
High birth weight may also impact the baby in the long term as studies have shown associations with increased risk of overweight, obesity, and type 2 diabetes mellitus.[4][16][17] Studies have shown that the long-term overweight risk is doubled when the birth weight is greater than 4,000 g. The risk of type 2 diabetes mellitus as an adult is 19% higher in babies weighing more than 4,500 g at birth compared to those with birth weights between 4,000 g and 4,500 g.[18]
Pregnant mother complications
editComplications of the pregnant mother include: emergency cesarean section, postpartum hemorrhage, and obstetric anal sphincter injury.[7] Compared to pregnancies without macrosomia, pregnant women giving birth to newborns weighing between 4,000 grams and 4,500 grams are at two times greater risk of complications, and those giving birth to infants over 4,500 grams are at three times greater risk.[7]
Causes
editMultiple factors have been shown to increase likelihood of infant macrosomia, including preexisting obesity, diabetes, or dyslipidemia of the mother, gestational diabetes, post-term pregnancy, prior history of a macrosomic birth, genetics, and other factors.[4]
Risk factors
editDiabetes of the mother
editOne of the primary risk factors of LGA births and macrosomia is poorly-controlled maternal diabetes, particularly gestational diabetes (GD), as well as preexisting type 2 diabetes mellitus (DM).[19] The risk of having a macrosomic fetus is three times greater in mothers with diabetes than those without diabetes.[20]
Obesity in the mother
editObesity prior to pregnancy and maternal weight gain above recommended guidelines during pregnancy are another key risk factor for macrosomia or LGA infants.[21][22][23] It has been demonstrated that while maternal obesity and gestational diabetes are independent risk factors for LGA and macrosomia, they can act synergistically, with even higher risk of macrosomia when both are present.[4][20]
Genetics
editGenetics can also play a role in having an LGA baby and it is seen that taller, heavier parents tend to have larger babies.[24] Genetic disorders of overgrowth (e.g. Beckwith–Wiedemann syndrome, Sotos syndrome, Perlman syndrome, Simpson-Golabi-Behmel syndrome) are often characterized by macrosomia.[25][26]
Other risk factors
edit- Gestational age: pregnancies that go beyond 40 weeks increase incidence of an LGA infant[20]
- Fetal sex: male infants tend to weigh more than female infants[8]
- Multiparity: giving birth to previous LGA infants vs. non-LGA infants[8]
- Frozen embryo transfer as fertility treatment, as compared with fresh embryo transfer or no artificial assistance[27][28]
Mechanism
editHow each of these factors leads to excess fetal growth is complex and not completely understood.[4][29]
Traditionally, the Pedersen hypothesis has been used to explain the mechanism in which uncontrolled gestational diabetes can lead to macrosomia, and many aspects of it have been confirmed with further studies.[20] This explanation proposes that impaired glucose control in the mother leads to a hyperglycemic state for the fetus, which leads to a hyperinsulinemia response, in turn causing increased glucose metabolism, fat deposition, and excess growth.[20][29][30]
It has also been shown that different patterns of excess fetal growth are seen in diabetic associated macrosomia compared to other predisposing factors, suggesting different underlying mechanisms.[4][29] Specifically, macrosomic infants associated with glucose abnormalities are seen to have increased body fat, larger shoulders and abdominal circumference.[4][29]
Diagnosis
editDiagnosing fetal macrosomia cannot be performed until after birth, as evaluating a baby's weight in the womb may be inaccurate.[20] While ultrasound has been the primary method for diagnosing LGA, this form of fetal weight assessment remains imprecise, as the fetus is a highly variable structure in regards to density and weight— no matter the gestational age.[20] Ultrasonography involves an algorithm that incorporates biometric measurements of the fetus, such as biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL), to calculate the estimated fetal weight (EFW).[31] Variability of fetal weight estimations has been linked to differences due to sensitivity and specificity of ultrasound algorithms as well as to the individual performing the ultrasound examination.[32]
In addition to sonography, fetal weight can also be assessed using clinical and maternal methods. Clinical methods for estimating fetal weight involves measuring the mother's symphysis-fundal height and performing Leopold's maneuvers, which can help with determining the fetus position in utero in addition to size.[32] However, as this method relies heavily on practitioner experience and technique, it does not provide an accurate and definite diagnosis of an LGA infant and would only serve as a potential indication of suspected macrosomia.[32] Fetal weight can also be estimated through a mother's subjective assessment of the fetus size, but this method is dependent on a mother's experience with past pregnancies and may not be clinically useful.[32] There are new methods being studied for their accuracy in predicting fetal weight, such as measuring fetal soft tissue, but more research needs to be done to find a consistent, reliable method.[33]
Prevention
editLGA and fetal macrosomia associated with poor glycemic control can be prevented by effective blood glucose management below a mean blood glucose level of 100 mg/dl before and during pregnancy; additionally, closely monitoring weight gain and diet during pregnancy can help to prevent LGA and fetal macrosomia.[34][35] Women with obesity that undergo weight loss can greatly decrease their chances of having a macrosomic or LGA infant.[35] Additionally, regular prenatal care and routine check-ups with one's physician are important in planning pregnancy, especially if one has obesity, diabetes, hypertension, or other conditions before conception.[34]
Screening
editMost screening for LGA and macrosomia occurs during prenatal check-ups, where both fundal height and ultrasound scans can give an approximate measurement of the baby's proportions.[36] Two-dimensional ultrasound can be used to screen for macrosomia and LGA but estimations are generally not precise at any gestational age until birth.[35]
Management
editAn approach that is sometimes suggested is to induce labor close to the estimated due date (at term) or near that date. The rationale is that by the baby being born with a lower birth weight, there would be a lower risk of long labors, cesarean section, bone fractures, and shoulder dystocia.[10] However, this method could increase the number of women with perineal tears, and failed inductions can prompt the need for emergency cesarean sections.[10] There is not strong evidence that an induced birth increases the risk of the person requiring a cesarean section. Another consideration is that since there may be inaccuracies in estimating or predicting the neonates weight in utero, there is a risk of inducing labor unnecessarily.[10] Doctors disagree whether women should be induced for suspected macrosomia and more research is needed to find out what is best for women and their babies.[10]
Elective cesarean section has also been presented as a potential delivery method for infants of suspected macrosomia, as it can serve to prevent possible birth trauma. However, the American College of Obstetricians and Gynecologists recommends that cesarean delivery should only be considered if the fetus is an estimated weight of at least 5,000 grams in non-diabetic mothers and at least 4,500 grams in diabetic mothers.[37] A number needed to treat analysis determined that approximately 3,700 women with suspected fetal macrosomia would have to undergo an unnecessary cesarean section in order to prevent one incident of brachial plexus injuries secondary to shoulder dystocia.[8]
Management of gestational diabetes through dietary modifications and anti-diabetic medications has been shown to decrease the incidence of LGA.[38] The use of metformin to control maternal blood glucose levels has shown to be more effective than using insulin alone in reducing the likelihood of fetal macrosomia.[39] There is a 20% lower chance of having an LGA baby when using metformin to manage diabetes compared to using insulin.[40]
Modifiable risk factors that increase the incidence of LGA births, such as gestational weight gain above recommended BMI guidelines, can be managed with lifestyle modifications, including maintaining a balanced diet and exercising.[41][42] Such interventions can help mothers achieve the recommended gestational weight and lower the incidence of fetal macrosomia in obese and overweight women.[41][42] The World Health Organization also recommends that mothers aim for their recommended BMI prior to conception.[23] In general, obese mothers or women with excessive gestational weight gain may have higher risk of pregnancy complications (ranging from LGA, shoulder dystocia, etc.).[43]
Epidemiology
editIn healthy pregnancies without pre-term or post-term health complications, fetal macrosomia has been observed to affect around 12% of newborns.[10] By comparison, women with gestational diabetes are at an increased risk of giving birth to LGA babies, where ~15-45% of neonates may be affected.[10] In 2017, the National Center of Health Statistics found that 7.8% of live-born infants born in the United States meet the definition of macrosomia, where their birth weight surpasses the threshold of 4000 grams (about 8.8 pounds).[10] Women in Europe and the United States tend to have higher pre-term body weight and have increased gestational weight during pregnancy compared to women in east Asia.[44] Thus, women in Europe and the United States, with higher gestational weight gain, tend to have higher associated risk of LGA infants, macrosomia and cesarean.[44] In European countries, the prevalence of births of newborns weighing between 4,000 g and 4,499 g is 8% to 21%, and in Asian countries the prevalence is between 1% and 8%.[45] In general, rates of LGA infants have increased 15-25% in many countries including the United States, Canada, Germany, Denmark, Scotland and more in the past 20–30 years, suggesting an increase in LGA births worldwide.[46]
References
edit- ^ Szmyd B, Biedrzycka M, Karuga FF, Strzelecka I, Respondek-Liberska M (2021). "Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia". Journal of Clinical Medicine. 10 (949): 949. doi:10.3390/jcm10050949. PMC 7957748. PMID 33804406.
- ^ Henriksen T (2008). "The macrosomic fetus: a challenge in current obstetrics". Acta Obstetricia et Gynecologica Scandinavica. 87 (2): 134–45. doi:10.1080/00016340801899289. PMID 18231880. S2CID 38118355.
- ^ McGrath RT, Glastras SJ, Hocking SL, Fulcher GR (August 2018). "Large-for-Gestational-Age Neonates in Type 1 Diabetes and Pregnancy: Contribution of Factors Beyond Hyperglycemia". Diabetes Care. 41 (8): 1821–1828. doi:10.2337/dc18-0551. PMID 30030258. S2CID 207369659.
- ^ a b c d e f g h Barth Jr WH, Jackson R, et al. (Committee on Practice Bulletins—Obstetrics) (January 2020). "Macrosomia: ACOG Practice Bulletin, Number 216". Obstetrics and Gynecology. 135 (1): e18–e35. doi:10.1097/AOG.0000000000003606. PMID 31856124.
- ^ a b c Boulet SL, Alexander GR, Salihu HM, Pass M (May 2003). "Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk". American Journal of Obstetrics and Gynecology. 188 (5): 1372–8. doi:10.1067/mob.2003.302. PMID 12748514.
- ^ a b "Fetal macrosomia - Symptoms and causes". Mayo Clinic. Retrieved 2021-09-15.
- ^ a b c Beta J, Khan N, Khalil A, Fiolna M, Ramadan G, Akolekar R (September 2019). "Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis". Ultrasound in Obstetrics & Gynecology. 54 (3): 308–318. doi:10.1002/uog.20279. PMID 30938004.
- ^ a b c d Zamorski MA, Biggs WS (January 2001). "Management of suspected fetal macrosomia". American Family Physician. 63 (2): 302–6. PMID 11201695.
- ^ a b Lapunzina P, Camelo JS, Rittler M, Castilla EE (February 2002). "Risks of congenital anomalies in large for gestational age infants". The Journal of Pediatrics. 140 (2): 200–4. doi:10.1067/mpd.2002.121696. PMID 11865271.
- ^ a b c d e f g h Boulvain, Michel; Thornton, Jim G. (2023-03-08). "Induction of labour at or near term for suspected fetal macrosomia". The Cochrane Database of Systematic Reviews. 2023 (3): CD000938. doi:10.1002/14651858.CD000938.pub3. ISSN 1469-493X. PMC 9995561. PMID 36884238.
- ^ a b Young BC, Ecker JL (February 2013). "Fetal macrosomia and shoulder dystocia in women with gestational diabetes: risks amenable to treatment?". Current Diabetes Reports. 13 (1): 12–8. doi:10.1007/s11892-012-0338-8. PMID 23076441. S2CID 4385185.
- ^ a b Rouse DJ, Owen J, Goldenberg RL, Cliver SP (November 1996). "The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound". JAMA. 276 (18): 1480–6. doi:10.1001/jama.1996.03540180036030. PMID 8903259.
- ^ Morrison JC, Sanders JR, Magann EF, Wiser WL (December 1992). "The diagnosis and management of dystocia of the shoulder". Surgery, Gynecology & Obstetrics. 175 (6): 515–22. PMID 1448731.
- ^ Rozance PJ (February 2014). "Update on neonatal hypoglycemia". Current Opinion in Endocrinology, Diabetes and Obesity. 21 (1): 45–50. doi:10.1097/MED.0000000000000027. PMC 4012366. PMID 24275620.
- ^ Mayer C, Joseph KS (February 2013). "Fetal growth: a review of terms, concepts and issues relevant to obstetrics". Ultrasound in Obstetrics & Gynecology. 41 (2): 136–45. doi:10.1002/uog.11204. PMID 22648955. S2CID 13304341.
- ^ Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C (October 2005). "Being big or growing fast: systematic review of size and growth in infancy and later obesity". BMJ. 331 (7522): 929. doi:10.1136/bmj.38586.411273.E0. PMC 1261184. PMID 16227306.
- ^ Schellong K, Schulz S, Harder T, Plagemann A (2012). Hernandez AV (ed.). "Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally". PLOS ONE. 7 (10): e47776. Bibcode:2012PLoSO...747776S. doi:10.1371/journal.pone.0047776. PMC 3474767. PMID 23082214.
- ^ Knop MR, Geng TT, Gorny AW, Ding R, Li C, Ley SH, Huang T (December 2018). "Birth Weight and Risk of Type 2 Diabetes Mellitus, Cardiovascular Disease, and Hypertension in Adults: A Meta-Analysis of 7 646 267 Participants From 135 Studies". Journal of the American Heart Association. 7 (23): e008870. doi:10.1161/JAHA.118.008870. PMC 6405546. PMID 30486715.
- ^ Leipold H, Worda C, Gruber CJ, Kautzky-Willer A, Husslein PW, Bancher-Todesca D (August 2005). "Large-for-gestational-age newborns in women with insulin-treated gestational diabetes under strict metabolic control". Wiener Klinische Wochenschrift. 117 (15–16): 521–5. doi:10.1007/s00508-005-0404-1. PMID 16160802. S2CID 7465313.
- ^ a b c d e f g Kc K, Shakya S, Zhang H (2015). "Gestational diabetes mellitus and macrosomia: a literature review". Annals of Nutrition & Metabolism. 66 (Suppl. 2): 14–20. doi:10.1159/000371628. PMID 26045324. S2CID 6536421.
- ^ Boubred F, Pauly V, Romain F, Fond G, Boyer L (2020-06-05). Farrar D (ed.). "The role of neighbourhood socioeconomic status in large for gestational age". PLOS ONE. 15 (6): e0233416. Bibcode:2020PLoSO..1533416B. doi:10.1371/journal.pone.0233416. PMC 7274403. PMID 32502147.
- ^ Henriksen T (February 2008). "The macrosomic fetus: a challenge in current obstetrics". Acta Obstetricia et Gynecologica Scandinavica. 87 (2): 134–45. doi:10.1080/00016340801899289. PMID 18231880. S2CID 38118355.
- ^ a b Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. (June 2017). "Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis". JAMA. 317 (21): 2207–2225. doi:10.1001/jama.2017.3635. PMC 5815056. PMID 28586887.
- ^ Christian M, Gustafsson J (2017). Pediatrik (2 uppl ed.). Liber. ISBN 9789147112968. OCLC 1001668564.
- ^ "Beckwith-Wiedemann syndrome". Genetics Home Reference. Retrieved 2020-07-30.
- ^ Vora N, Bianchi DW (October 2009). "Genetic considerations in the prenatal diagnosis of overgrowth syndromes". Prenatal Diagnosis. 29 (10): 923–9. doi:10.1002/pd.2319. PMC 4426974. PMID 19609940.
- ^ Berntsen S, Pinborg A (May 2018). "Large for gestational age and macrosomia in singletons born after frozen/thawed embryo transfer (FET) in assisted reproductive technology (ART)". Birth Defects Research. 110 (8): 630–643. doi:10.1002/bdr2.1219. PMID 29714057. S2CID 25437950.
- ^ Orvieto R, Kirshenbaum M, Gleicher N (2020). "Is Embryo Cryopreservation Causing Macrosomia-and What Else?". Frontiers in Endocrinology. 11: 19. doi:10.3389/fendo.2020.00019. PMC 6997460. PMID 32047479.
- ^ a b c d Avery's diseases of the newborn. Christine A. Gleason, Sherin U. Devaskar, Mary Ellen Avery (9th ed.). Philadelphia, PA: Elsevier/Saunders. 2012. ISBN 978-1-4557-2714-8. OCLC 768412368.
{{cite book}}: CS1 maint: others (link) - ^ Olmos, Pablo; Martelo, Grettel; Reimer, Verena; Rigotti, Attilio; Busso, Dolores; Belmar, Cristián; González, Rogelio; Goldenberg, Denisse; Samith, Bárbara; Santos, José-Luis; Escalona, Manuel (November 2013). "[Nutrients other than glucose might explain fetal overgrowth in gestational diabetic pregnancies]". Revista Médica de Chile. 141 (11): 1441–1448. doi:10.4067/S0034-98872013001100011. ISSN 0717-6163. PMID 24718471.
- ^ Coomarasamy A, Connock M, Thornton J, Khan KS (November 2005). "Accuracy of ultrasound biometry in the prediction of macrosomia: a systematic quantitative review". BJOG. 112 (11): 1461–6. doi:10.1111/j.1471-0528.2005.00702.x. PMID 16225563. S2CID 34330598.
- ^ a b c d Ray EM, Alhusen JL (2016). "The Suspected Macrosomic Fetus at Term: A Clinical Dilemma". Journal of Midwifery & Women's Health. 61 (2): 263–9. doi:10.1111/jmwh.12414. PMID 26869131.
- ^ Warska A, Maliszewska A, Wnuk A, Szyszka B, Sawicki W, Cendrowski K (March 2018). "Current knowledge on the use of ultrasound measurements of fetal soft tissues for the assessment of pregnancy development". Journal of Ultrasonography. 18 (72): 50–55. doi:10.15557/JoU.2018.0008. PMC 5911719. PMID 29844941.
- ^ a b "Large for Gestational Age Babies: Reasons, Signs & Treatment". parenting.firstcry.com. Retrieved 2021-09-15.
- ^ a b c "UpToDate". www.uptodate.com. Retrieved 2021-09-15.
- ^ Silasi, Michelle (2018-01-01). "Fetal Macrosomia". Obstetric Imaging: Fetal Diagnosis and Care: 460–462.e1. doi:10.1016/B978-0-323-44548-1.00108-X. ISBN 9780323445481.
- ^ "Safe Prevention of the Primary Cesarean Delivery". www.acog.org. Archived from the original on 2023-02-04. Retrieved 2020-08-04.
- ^ Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L (July 2013). "Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research". Annals of Internal Medicine. 159 (2): 123–9. doi:10.7326/0003-4819-159-2-201307160-00661. PMID 23712381. S2CID 21881403.
- ^ Guo L, Ma J, Tang J, Hu D, Zhang W, Zhao X (2019). "Comparative Efficacy and Safety of Metformin, Glyburide, and Insulin in Treating Gestational Diabetes Mellitus: A Meta-Analysis". Journal of Diabetes Research. 2019: 9804708. doi:10.1155/2019/9804708. PMC 6875019. PMID 31781670.
- ^ Butalia S, Gutierrez L, Lodha A, Aitken E, Zakariasen A, Donovan L (January 2017). "Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis". Diabetic Medicine. 34 (1): 27–36. doi:10.1111/dme.13150. PMID 27150509. S2CID 3418227.
- ^ a b World Health Organization (2016). WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, Switzerland. ISBN 978-92-4-154991-2. OCLC 974355266.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b "Weight Gain During Pregnancy | Pregnancy | Maternal and Infant Health C". www.cdc.gov. 2019-01-17. Retrieved 2020-08-04.
- ^ Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. (July 2019). "Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts". BJOG. 126 (8): 984–995. doi:10.1111/1471-0528.15661. PMC 6554069. PMID 30786138.
- ^ a b Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. (August 2018). "Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women". BMC Medicine. 16 (1): 153. doi:10.1186/s12916-018-1128-1. PMC 6117916. PMID 30165842.
- ^ Culliney KA, Parry GK, Brown J, Crowther CA, et al. (Cochrane Pregnancy and Childbirth Group) (April 2016). "Regimens of fetal surveillance of suspected large-for-gestational-age fetuses for improving health outcomes". The Cochrane Database of Systematic Reviews. 2016 (4): CD011739. doi:10.1002/14651858.CD011739.pub2. PMC 7081118. PMID 27045604.
- ^ Miller V, Saxena S, Farhan M (2010). "Management of large-for-gestational-age pregnancy in non-diabetic women". The Obstetrician & Gynaecologist. 12 (4): 250–256. doi:10.1576/toag.12.4.250.27617.