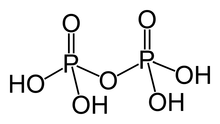
Pyrophosphoric acid, also known as diphosphoric acid, is the inorganic compound with the formula H4P2O7 or, more descriptively, [(HO)2P(O)]2O. Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes in two polymorphs, which melt at 54.3 and 71.5 °C. The compound is a component of polyphosphoric acid, an important source of phosphoric acid.[1] Anions, salts, and esters of pyrophosphoric acid are called pyrophosphates.
| |

| |
| Names | |
|---|---|
| IUPAC names
Diphosphoric acid
μ-oxido-bis(dihydroxidooxidophosphorus) | |
| Other names
Pyrophosphoric acid
Phosphonophosphoric acid Phosphono dihydrogenphosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.017.795 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H4P2O7 | |
| Molar mass | 177.97 g/mol |
| Melting point | 71.5 °C (160.7 °F; 344.6 K) |
| Extremely soluble | |
| Solubility | Very soluble in alcohol, ether |
| Conjugate base | Pyrophosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editIt can be prepared by reaction of phosphoric acid with phosphoryl chloride:[2]
- 5 H3PO4 + POCl3 → 3 H4P2O7 + 3 HCl
It can also be prepared by ion exchange from sodium pyrophosphate or by treating lead pyrophosphate with hydrogen sulfide.[1]
Boiling the water from orthophosphoric acid will not dehydrate it to pure pyrophosphoric acid, instead a mixture of ortho, pyro, and polyphosphoric acids are produced, the maximum pyrophosphoric acid concentration remains below 50% and occurs slightly before what would otherwise be pure pyrophosphoric acid.[3]
Reactions
editPyrophosphoric acid is a tetraprotic acid, with four distinct pKa's:[4]
- H4P2O7 ⇌ [H3P2O7]− + H+, pKa = 0.85
- [H3P2O7]− ⇌ [H2P2O7]2− + H+, pKa = 1.96
- [H2P2O7]2− ⇌ [HP2O7]3− + H+, pKa = 6.60
- [HP2O7]3− ⇌ [P2O7]4− + H+, pKa = 9.41
The pKa's occur in two distinct ranges because deprotonations occur on separate phosphate groups. For comparison with the pKa's for phosphoric acid are 2.14, 7.20, and 12.37.
At physiological pH's, pyrophosphate exists as a mixture of doubly and singly protonated forms.
When molten, pyrophosphoric acid rapidly converts to an equilibrium mixture of phosphoric acid, pyrophosphoric acid and polyphosphoric acids. The percentage by weight of pyrophosphoric acid is around 40% and it is difficult to recrystallise from the melt.
Even in cold water, pyrophosphoric acid hydrolyses to phosphoric acid. All polyphosphoric acids behave similarly.[5]
- H4P2O7 + H2O → 2 H3PO4
Safety
editWhile pyrophosphoric acid is corrosive, it is not known to be otherwise toxic.[6]
History
editThe name pyrophosphoric acid was given by a "Mr. Clarke of Glasgow" in 1827 who is credited with its discovery following the heating to red heat of a sodium phosphate salt. It was found that phosphoric acid when heated to red heat formed pyrophosphoric acid that was readily converted to phosphoric acid by hot water.[7]
See also
editReferences
edit- ^ a b Havelange, Sébastien; Lierde, Nicolas; Germeau, Alain; Martins, Emmanuel; Theys, Tibaut; Sonveaux, Marc; Toussaint, Claudia; Schrödter, Klaus; Bettermann, Gerhard; Staffel, Thomas; Wahl, Friedrich; Klein, Thomas; Hofmann, Thomas (2022). "Phosphoric Acid and Phosphates". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–55. doi:10.1002/14356007.a19_465.pub4. ISBN 9783527303854. S2CID 246266565.
- ^ R. Klement (1963). "Condensed Orthophosphates". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2. NY, NY: Academic Press. p. 546.
- ^ Korte, Carsten; Conti, Fosca; Wackerl, Jürgen; Lehnert, Werner (2016), Li, Qingfeng; Aili, David; Hjuler, Hans Aage; Jensen, Jens Oluf (eds.), "Phosphoric Acid and its Interactions with Polybenzimidazole-Type Polymers", High Temperature Polymer Electrolyte Membrane Fuel Cells, Cham: Springer International Publishing, pp. 169–194, doi:10.1007/978-3-319-17082-4_8, ISBN 978-3-319-17081-7, retrieved 2023-02-11
- ^ Yadav, Prerna; Blacque, Olivier; Roodt, Andreas; Zelder, Felix (2021). "Induced fit activity-based sensing: A mechanistic study of pyrophosphate detection with a "flexible" Fe-salen complex". Inorganic Chemistry Frontiers. 8 (19): 4313–4323. doi:10.1039/d1qi00209k. PMC 8477187. PMID 34603734.
- ^ Corbridge, D. (1995). "Chapter 3: Phosphates". Studies in inorganic Chemistry vol. 20. Elsevier Science B.V. pp. 169–305. doi:10.1016/B978-0-444-89307-9.50008-8. ISBN 0-444-89307-5.
- ^ Material Safety Data Sheet: Pyrophosphoric acid MSDS www.sciencelab.com
- ^ Beck, Lewis Caleb (1834). A Manual of Chemistry: Containing a Condensed View of the Present State of the Science, with Copious References to More Extensive Treatises, Original Papers, Etc. E.W & C Skinner. p. 160. Retrieved January 30, 2015.