3-Fluoroamphetamine (3-FA; PAL-353) is a stimulant drug from the amphetamine family which acts as a monoamine releaser with similar potency to methamphetamine but more selectivity for dopamine and norepinephrine release over serotonin.[3] It is self-administered by mice to a similar extent to related drugs such as 4-fluoroamphetamine and 3-methylamphetamine.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | 3FPPA |
| Addiction liability | moderate[1] |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | 20 - 60 minutes |
| Elimination half-life | 90 minutes |
| Duration of action | 2 - 3 hours "3-FA". Psychonautwiki.[unreliable medical source?] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H12FN |
| Molar mass | 153.200 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.0 [2] g/cm3 |
| Boiling point | 208.2[2] °C (406.8 °F) |
| |
| |
| | |
3-Fluoroamphetamine often found its use as a designer drug in several studies to mimic the effects of illegal amphetamines.[5] It has also appeared on the drug market for recreational use as an amphetamine alternative, its has been reported in January 2009 to the European Early Warning System by Belgium. Little is known about the exact history of this compound.[6]
Chemistry
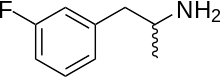
edit3-Fluoroamphetamine is a synthetic molecule of the substituted amphetamine class. Molecules in this class contain a phenethylamine core that consists of a phenyl ring, ethyl chain, a terminal amino (NH2) group and a methyl substitution at Rα. Amphetamines themselves belong to the class of alpha-methylated phenethylamines.[citation needed] Substituted amphetamines can be synthesised by substituting a hydrogen atom with a substituent which in 3-fluoroamphetamines case is a fluorine atom positioned on the third carbon of the phenyl ring.3-Fluoroamphetamine has a chemical formula of C9H12FN. At room temperature it is a liquid with molecular mass of 153.200 g·mol−1.[7]
Pharmacology
edit3-Fluoroamphetamine is a locomotor stimulant that acts as a substrate-based releaser, with selectivity for dopamine over serotonin. This rank order is about the same as for amphetamine when tested in non-human primates.[8] The halflife was also comparable to amphetamine when tested in rats, namely 91 minutes. 3-Fluoroamphetamine is a good candidate for transdermal infusion since it is a relatively small molecule with a low melting point, has a weak basicity (pH of 9.97) and is moderately lipophilic. These properties make it easier to get through the stratum corneum, which is the lipid-rich outermost barrier of the skin.[9] By replacing a hydrogen in the ring by a fluor group, the compound is more likely to pass the blood brain barrier, making it easier to get into the brain. The P450 oxidase metabolism will most likely oxidise the 3-fluoroamphetamine in the 4 position, creating 3-fluoro-4-hydroxyamphetamine which is hypothesised to be aversive to the intake of stimulants.[10]
Molecular mechanism
editLike other amphetamine derivatives, 3-fluoroamphetamine acts as a monoamine releaser with a higher selectivity for dopamine and norepinephrine over serotonin.[11] There are multiple targets at which amphetamines can disrupt the normal function of these neurotransmitters. First, 3-fluoroamphetamine can interact with their respective transmembrane monoamine transporters DAT, NET, and SERT to mediate neurotransmitter release and reuptake.[10] It does this by blocking these transmembrane transporters, which usually transport monoamines back into the presynapse from the synaptic cleft. Blocking of DAT, NET, and SERT causes prolonged elevated concentrations of dopamine, norepinephrine, and serotonin in the synaptic cleft, causing more stimulant effects associated with these neurotransmitters.[citation needed]
Another way for amphetamine derivatives to influence neurotransmission is by entering the presynapse via DAT, NET, and SERT, where the amphetamine derivative accumulates inside the neuron and replaces monoamines in synaptic vesicles by interacting with vesicular monoamine transporter VMAT2. The concentration of free monoamines in the presynapse increases, prohibiting the inward transport of monoamines and encouraging the outward transport. Furthermore, amphetamine derivatives inhibit the action of mitochondrial monoamine oxidases (MOA), which catalyze the degradation of cytosolic monoamines. Inhibition thus increases cytosolic concentrations of monoamines even more.[12]
The mechanism of action for 3-fluoroamphetamine has not been individually studied yet, but various sources suggest that amphetamine derivatives generally have the same mechanism of action in monoaminergic neurons .[12] However, further investigation on 3-fluoroamphetamine could reveal more specific mechanisms in which this amphetamine derivative modulates processes within the body.
Uses
edit3-Fluoroamphetamine is distinguished by its potent stimulant and mild entactogenic effects, setting it apart from other amphetamines such as 4-FA, 2-FA, and 2-FMA. It lacks the productivity and focus-enhancing properties reported for 2-FA and 2-FMA, potentially limiting its appeal and availability.[citation needed] Beyond its recreational use, 3-fluoroamphetamine has been identified as a nonselective inhibitor of dopamine and serotonin reuptake, with research suggesting its potential to inhibit cancer cell proliferation in vitro and promote the release of growth factors in neural contexts.[13][14] These findings point to its utility in neuropharmacological research and possible applications in treating obesity, cancer, and cocaine dependency.[14][1]
Legal status
editChina
editAs of October 2015 3-fluoroamphetamine is a controlled substance in China.[15]
Germany
edit3-Fluoroamphetamine is controlled under the NpSG (New Psychoactive Substances Act)[16] as of November 26, 2016.[17] Production and import with the aim to place it on the market, administration to another person and trading is punishable.
New Zealand
3-Fluoroamphetamine is an amphetamine analogue, so is a Schedule 3 Class C controlled substance in New Zealand.[18]
Switzerland
3-Fluoroamphetamine is a controlled substance specifically named under Verzeichnis E.[19]
Turkey
3-Fluoroamphetamine is a classed as drug and is illegal to possess, produce, supply, or import.[20]
United Kingdom
3-Fluoroamphetamine is considered a Class A drug as a result of the amphetamine analogue clause of the Misuse of Drugs Act 1971.[21]
United States: 3-Fluoroamphetamine may be considered to be an analog of amphetamine, thus falling under the Federal Analogue Act. The Federal Analogue Act, 21 U.S.C. § 813, is a section of the United States Controlled Substances Act, allowing any chemical "substantially similar" to an illegal drug (in Schedule I or II) to be treated as if it were also in Schedule I or II, but only if it is intended for human consumption.
See also
edit- 2-Fluoroamphetamine (2-FA)
- 3-Fluoroethamphetamine (3-FEA)
- 3-Fluoromethamphetamine (3-FMA)
- 3-Hydroxyamphetamine (Gepefrine)
- 3-Methylamphetamine (3-MA)
- 3-Methoxyamphetamine (3-MeOA)
- 3-Trifluoromethylamphetamine (Norfenfluramine)
- 4-Fluoroamphetamine (4-FA)
- 3,4-Difluoroamphetamine
References
edit- ^ a b Puri A, Murnane KS, Blough BE, Banga AK (August 2017). "Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydrochloride". International Journal of Pharmaceutics. 528 (1–2): 452–462. doi:10.1016/j.ijpharm.2017.06.041. PMID 28633107.
- ^ a b "3-Fluoroamphetamine | C9H12FN". Chemspider. 2022.
- ^ Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB (February 2007). "Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 320 (2): 627–36. doi:10.1124/jpet.106.107383. PMID 17071819. S2CID 8326027.
- ^ Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL (May 2005). "Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs". The Journal of Pharmacology and Experimental Therapeutics. 313 (2): 848–54. doi:10.1124/jpet.104.080101. PMID 15677348. S2CID 12135483.
- ^ Seibert E, Mader E, Schmid MG (November 2021). "A simple and isocratic protein-based high performance liquid chromatography method for the enantioseparation of amphetamine derivatives". Journal of Chromatography Open. 1: 100013. doi:10.1016/j.jcoa.2021.100013. ISSN 2772-3917.
- ^ "GLOBAL SMART UPDATE 2009 Volume 2" (PDF). www.unodc.org. United Nations Office on Drugs and Crime. October 1, 2009.
- ^ "1-(3-Fluorophenyl)propan-2-amine 3D-FF36292". CymitQuimica. Retrieved 2024-03-14.
- ^ Kimmel HL, Manvich DF, Blough BE, Negus SS, Howell LL (December 2009). "Behavioral and neurochemical effects of amphetamine analogs that release monoamines in the squirrel monkey". Pharmacology, Biochemistry, and Behavior. 94 (2): 278–284. doi:10.1016/j.pbb.2009.09.007. PMC 2763934. PMID 19766133.
- ^ Jiang Y, Ray A, Junaid MS, Bhattaccharjee SA, Kelley K, Banga AK, et al. (February 2020). "The pharmacokinetics of 3-fluoroamphetamine following delivery using clinically relevant routes of administration". Drug Delivery and Translational Research. 10 (1): 271–281. doi:10.1007/s13346-019-00685-4. PMC 6982562. PMID 31642004.
- ^ a b Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA (April 2021). "Designer drugs: a medicinal chemistry perspective (II)". Annals of the New York Academy of Sciences. 1489 (1): 48–77. doi:10.1111/nyas.14349. PMID 32396701.
- ^ Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB (February 2007). "Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 320 (2): 627–636. doi:10.1124/jpet.106.107383. PMID 17071819.
- ^ a b Sitte HH, Freissmuth M (January 2015). "Amphetamines, new psychoactive drugs and the monoamine transporter cycle". Trends in Pharmacological Sciences. 36 (1): 41–50. doi:10.1016/j.tips.2014.11.006. PMC 4502921. PMID 25542076.
- ^ Gao Y, Du L, Li Q, Li Q, Zhu L, Yang M, et al. (July 2022). "How physical techniques improve the transdermal permeation of therapeutics: A review". Medicine. 101 (26): e29314. doi:10.1097/MD.0000000000029314. PMC 9239599. PMID 35777055.
- ^ a b "FF36292 1-(3-Fluorophenyl)propan-2-amine Datasheets". www.biosynth.com. Retrieved 2024-03-14.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- ^ "Anlage NpSG - Einzelnorm". www.gesetze-im-internet.de (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved 2024-03-14.
- ^ "Bundesgesetzblatt BGBl. Online-Archiv 1949 - 2022 | Bundesanzeiger Verlag" (PDF). www.bgbl.de (in German). Retrieved 2024-03-14.
- ^ "Misuse of Drugs Act 1975 No 116". legislation.govt.nz. July 1, 2022.
- ^ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland].
- ^ "Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü". resmigazete.gov.tr (in Turkish). Retrieved 2024-03-14.
- ^ "Misuse of Drugs Act 1971".