This article may be too technical for most readers to understand. (April 2022) |
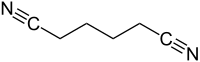
Adiponitrile is an organic compound with the chemical formula (CH2)4(CN)2. This viscous, colourless dinitrile is an important precursor to the polymer nylon 66. In 2005, about one million tonnes of adiponitrile were produced.[4]
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexanedinitrile[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1740005 | |
| ChemSpider | |
| ECHA InfoCard | 100.003.543 |
| EC Number |
|
| MeSH | adiponitrile |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2205 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8N2 | |
| Molar mass | 108.144 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 951 mg mL−1 |
| Melting point | 1 to 3 °C; 34 to 37 °F; 274 to 276 K |
| Boiling point | 295.1 °C; 563.1 °F; 568.2 K |
| 50 g/L (20 °C) | |
| Vapor pressure | 300 mPa (at 20 °C) |
Refractive index (nD)
|
1.438 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
84.5–85.3 kJ mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H301, H315, H319, H330, H335 | |
| P260, P284, P301+P310, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | 93 °C; 199 °F; 366 K (open cup)[2] |
| 550 °C (1,022 °F; 823 K) | |
| Explosive limits | 1.7–4.99% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
155 mg kg−1 (oral, rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[2] |
REL (Recommended)
|
TWA 4 ppm (18 mg/m3)[2] |
IDLH (Immediate danger)
|
N.D.[2] |
| Related compounds | |
Related alkanenitriles
|
Glutaronitrile |
Related compounds
|
hexanedioic acid hexanedihydrazide hexanedioyl dichloride hexanediamide 1,4-diisocyanobutane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Production
editEarly methods
editBecause of the industrial value of adiponitrile, many methods have been developed for its synthesis. Early industrial methods started from furfural and later by the chlorination of butadiene to give 1,4-dichloro-2-butene, which with sodium cyanide, converts to 3-hexenedinitrile, which in turn can be hydrogenated to adiponitrile:[4]
- ClCH2CH=CHCH2Cl + 2 NaCN → NCCH2CH=CHCH2CN + 2 NaCl
- NCCH2CH=CHCH2CN + H2 → NC(CH2)4CN
Adiponitrile has also been produced from adipic acid, by dehydration of the diamide, but this is rarely employed.
Modern methods
editAfter patent application in 2004, the majority of adiponitrile is prepared by the nickel-catalysed hydrocyanation of butadiene, as discovered at DuPont, pioneered by William C. Drinkard. The net reaction is:
- CH2=CHCH=CH2 + 2 HCN → NC(CH2)4CN
The process involves several stages, the first of which involves monohydrocyanation (the addition of one molecule of HCN), affording isomers of pentenenitriles as well as 2- and 3-methylbutanenitriles. These unsaturated nitriles are subsequently isomerized to the 3-and 4-pentenenitriles. In the final stage, these pentenenitriles are subjected to a second hydrocyanation, in an anti-Markovnikov sense, to produce adiponitrile.[4]
3-pentenenitrile, formed in the first hydrocyanation, can undergo alkene metathesis to give dicyanobutenes, which are readily hydrogenated as described above. A useful byproduct of the production of adiponitrile is 2-methylglutaronitrile.
The other major industrial method involves hydrodimerization, starting from acrylonitrile:[5][6]
- 2 CH2=CHCN + 2 e− + 2 H+ → NCCH2CH2CH2CH2CN
The electrolytic coupling of acrylonitrile was discovered at Monsanto Company.
Applications
editAlmost all adiponitrile is hydrogenated to hexane-1,6-diamine for the production of nylon:[7]
- NC(CH2)4CN + 4 H2 → H2N(CH2)6NH2
Like other nitriles, adiponitrile is susceptible to hydrolysis; however, the resulting adipic acid is less expensively prepared via other routes.
Production
editIn 2018, there existed approximately 1.5 million metric tons of capacity.[citation needed] The main producers of adiponitrile were:[8][9]
- Ascend Performance Materials: Decatur, Alabama (US); 400 metric kilotons per year (kt/y), expanded to 580 kt/y by 2022
- Invista: Victoria, Texas and Orange, Texas, (US)
- Invista and BASF "Butachimie ADN plant": Chalampé (France); production to be increased from 100 kt/y in 2020 to 600 kt/y
- Asahi Kasei (Japan)
BASF closed the 128 kt/y ADN plant at Seal Sands in 2009.[10]
In 2015, the Shandong Runxing New Material 100 kt/y plant suffered an explosion and was not reopened.[8] In 2022, Invista plans to open a 300–400 kt/y plant in Shanghai.[11]
Safety
editThe LD50 (median lethal dose) of adiponitrile is 300 mg/kg for oral ingestion by rats.[4]
In 1990, ACGIH adopted a time-weighted average Threshold Limit Value of 2ppm for work-related skin exposure.[12]
The NIOSH recommended skin exposure limit for a work-related time weighted average concentration is 4ppm (18 mg/m3).[13]
Adiponitrile is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[14]
References
edit- ^ "adiponitrile - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 15 June 2012.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0015". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Archived copy". Archived from the original on 2015-04-02. Retrieved 2015-03-26.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ a b c d M. T. Musser, "Adipic Acid" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a01_269
- ^ Cardoso, D. S.; Šljukić, B.; Santos, D. M.; Sequeira, C. A. (2017). "Organic Electrosynthesis: From Laboratorial Practice to Industrial Applications". Organic Process Research & Development. 21 (9) (published July 17, 2017): 1213–1226. doi:10.1021/acs.oprd.7b00004.
- ^ Baizer, Manuel M. (1964). "Electrolytic Reductive Coupling". Journal of the Electrochemical Society. 111 (2): 215. doi:10.1149/1.2426086.
- ^ Robert A. Smiley "Hexamethylenediamine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a12_629
- ^ a b Alexander, Tullo (7 Oct 2018). "The chemical industry is bracing for a nylon 6,6 shortage". cen.acs.org. 96 (40). Retrieved 2 March 2021.
- ^ "Ascend Finalizes $175 Million ADN Project in Alabama | CHEManager". www.chemanager-online.com. Retrieved 2 March 2021.
- ^ Gale, Lindsay (1 April 2009). "End of the line for Seal Sands". KHL Group. Retrieved 28 April 2023.
- ^ "INVISTA China ADN project receives final construction permit". invista.com. Retrieved 2 March 2021.
- ^ 2009 TLVs and BEIs, American Conference of Governmental Industrial Hygienists, Signature Publications, page 11 of 254.
- ^ NIOSH Pocket Guide NIOSH Publication 2005-149; September 2005
- ^ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.
{{cite journal}}: Cite journal requires|journal=(help)
External links
edit- International Chemical Safety Card 0211
- NIOSH Pocket Guide to Chemical Hazards. "#0015". National Institute for Occupational Safety and Health (NIOSH).
- www.chemicalland.com
- www.nist.gov
