In organic chemistry, a cyclitol is a cycloalkane containing at least three hydroxyl, each attached to a different ring carbon atom.[1] The general formula for an unsubstituted cyclitol is C
nH
2n-x(OH)
x or C
nH
2nO
x where 3 ≤ x ≤ n.
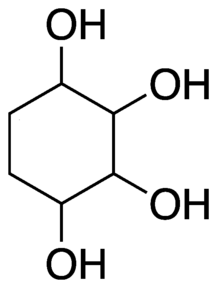
The name is also used for compounds that can be viewed as result of substituting various functional groups for the hydrogen atoms in such a molecule, as well as similar molecules with one or more double bonds in the ring.[citation needed]
Cyclitols and their derivatives are some of the compatible solutes which are formed in a plant as a response to salt or water stress. Some cyclitols (e.g. quinic or shikimic acid) are parts of hydrolysable tannins.
Isomerism and nomenclature
editUnsubstituted cyclitols with the same ring size and number of hydroxyls may exist in several structural isomers, depending on the position of the hydroxyls along the ring. For example, cyclohexanetriol exists in three distinct isomers (1,2,3-, 1,2,4-, and 1,3,5-).
Furthermore, the hydrogen and the hydroxyl on each carbon atom may lie in two possible arrangements relative to the local ring plane; so that each structural isomer may exist in several stereoisomers, depending on which side of the ring plane the hydroxyls are. For example, there are nine stereoisomers of 1,2,3,4,5,6-cyclohexanehexol (inositol), and two of them are enantiomers. The IUPAC has provided a nomenclature for cyclitol stereoisomers.[2]
Naturally occurring cyclitols
editUnsubstituted
edit- Conduritol, or cyclohex-5-ene-1,2,3,4-tetrol; two out of ten possible isomers.
- Inositol, or cyclohexane-1,2,3,4,5,6-hexol; four out of nine possible isomers.
- Cyclohexanetetrol[3]
Substituted
edit- Bornesitol; (1R,2R,3S,4S,5R,6S)-6-methoxycyclohexane-1,2,3,4,5-pentol; D-(−)-O-methyl-myo-inositol
- Pinitol; (1S,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol; 3-O-methyl-D-chiro-inositol
- Ononitol; (1R,2S,3S,4S,5S,6S)-6-methoxycyclohexane-1,2,3,4,5-pentaol; 4-O-methyl-myo-inositol
- Pinpollitol; (1R,2R,3R,4S,5R,6S)-3,6-dimethoxycyclohexane-1,2,4,5-tetraol; di-O-methyl-(+)-chiro-inositol
- Quebrachitol; (1R,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol; 2-0-methyl-chiro-inositol
- Quinic acid; (1S,3R,4S,5R)-1,3,4,5-tetrahydroxycyclohexanecarboxylic acid
- Shikimic acid; (3R,4S,5R)-3,4,5-trihydroxycyclohex-1-ene-1-carboxylic acid
- Valienol; (1S,2S,3S,4R)-5-(Hydroxymethyl)cyclohex-5-ene-1,2,3,4-tetrol
- Viscumitol (1R,2S,3R,4S,5R,6S)-5,6-dimethoxycyclohexane-1,2,3,4-tetraol; 1,2-di-O-methyl-muco-inositol
Glycosides
edit- Ciceritol, a pinitol digalactoside
Phosphates
edit- Phytic acid; (1R,2S,3r,4R,5S,6s)-cyclohexane-1,2,3,4,5,6-hexayl hexakis[dihydrogen(phosphate)]; inositol hexakisphosphate
Other cyclitols
editAnalysis methods
editIn 1955, Posternak and others described the separation of cyclitols by paper chromatography in various solvents, and three methods of development: Tollens reagents, the Meillère reagent (based on the Scherer-Gallois reaction), and digestion by Acetobacter suboxydans followed by Tollens reagent.[5]
See also
editReferences
edit- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Cyclitols". doi:10.1351/goldbook.C01493
- ^ CON and CBN IUPAC Commissions on Nomenclature (1968): "The Nomenclature of Cyclitols - Tentative Rules". European Journal of Biochemistry, volume 5, pages 1-12. doi:10.1111/j.1432-1033.1968.tb00328.x
- ^ J. S. Craigie (1969): "Some Salinity-Induced Changes in Growth, Pigments, and Cyclohexanetetrol Content of Monochrysis lutheri". Journal of the Fisheries Research Board of Canada, volume 26, issue 11, pages 2959-2967. doi:10.1139/f69-282
- ^ Nihat Akbulut and Metin Balci (1988): "A new and stereospecific synthesis of cyclitols: (1,2,4/3)-, (1,2/3,4)-, and (1,3/2,4)-cyclohexanetetrols". Journal of Organic Chemistry, volume 53, issue 14, pages 3338-3342. doi:10.1021/jo00249a039
- ^ Th. Posternak, D. Reymond, W. Haerdi (1955): "Recherches dans la série des cyclitols XX. Chromatographie sur papier de cyclitols et de cycloses". Helvetica Chimica Acta volume 38, issue 1, pages 191-194 doi:10.1002/hlca.19550380122