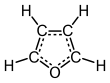
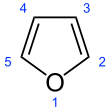
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Furan[1] | |||
| Systematic IUPAC name
1,4-Epoxybuta-1,3-diene 1-Oxacyclopenta-2,4-diene | |||
| Other names
Oxole
Oxa[5]annulene 1,4-Epoxy-1,3-butadiene 5-Oxacyclopenta-1,3-diene 5-Oxacyclo-1,3-pentadiene Furfuran Divinylene oxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 103221 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.390 | ||
| EC Number |
| ||
| 25716 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2389 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H4O | |||
| Molar mass | 68.075 g·mol−1 | ||
| Appearance | Colorless, volatile liquid | ||
| Density | 0.936 g/mL | ||
| Melting point | −85.6 °C (−122.1 °F; 187.6 K) | ||
| Boiling point | 31.3 °C (88.3 °F; 304.4 K) | ||
| -43.09·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H224, H302, H315, H332, H341, H350, H373, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P308+P313, P312, P314, P321, P330, P332+P313, P362, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −36 °C (−33 °F; 237 K) | ||
| 390 °C (734 °F; 663 K) | |||
| Explosive limits | Lower: 2.3% Upper: 14.3% at 20 °C | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
> 2 g/kg (rat) | ||
| Safety data sheet (SDS) | Pennakem | ||
| Related compounds | |||
Related heterocycles
|
Pyrrole Thiophene | ||
Related compounds
|
Tetrahydrofuran (THF) 2,5-Dimethylfuran Benzofuran Dibenzofuran | ||
| Structure | |||
| C2v | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water.[2] Its odor is "strong, ethereal; chloroform-like".[3] It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals.[4]
History
editThe name "furan" comes from the Latin furfur, which means bran[5] (furfural is produced from bran). The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limpricht in 1870, although he called it "tetraphenol" (as if it were a four-carbon analog to phenol, C6H5OH).[6][7]
Production
editIndustrially, furan is manufactured by the palladium-catalyzed decarbonylation of furfural, or by the copper-catalyzed oxidation of 1,3-butadiene:[4]
In the laboratory, furan can be obtained from furfural by oxidation to 2-furoic acid, followed by decarboxylation.[8] It can also be prepared directly by thermal decomposition of pentose-containing materials, and cellulosic solids, especially pine wood.
Synthesis of furans
editThe Feist–Benary synthesis is a classic way to synthesize furans. The reaction involves alkylation of 1,3-diketones with α-bromoketones followed by dehydration of an intermediate hydroxydihydrofuran.[9] The other traditional route involve the reaction of 1,4-diketones with phosphorus pentoxide (P2O5) in the Paal–Knorr synthesis.[10]
Many routes exist for the synthesis of substituted furans.[11][12]
- Furan in nature and commerce
-
The drug Zantac, also known as ranitidine.
-
Furfural, derived from sugars, is the major source of furans
-
methanofuran is a cofactor in methanogenesis.
Structure and bonding
editFuran has aromatic character because one of the lone pairs of electrons on the oxygen atom is delocalized into the ring, creating a 4n + 2 aromatic system (see Hückel's rule). The aromaticity is modest relative to that for benzene and related heterocycles thiophene and pyrrole. The resonance energies of benzene, pyrrole, thiophene, and furan are, respectively, 152, 88, 121, and 67 kJ/mol (36, 21, 29, and 16 kcal/mol). Thus, these heterocycles, especially furan, are far less aromatic than benzene, as is manifested in the lability of these rings.[13] The molecule is flat but the C=C groups attached to oxygen retain significant double bond character. The other lone pair of electrons of the oxygen atom extends in the plane of the flat ring system.
Examination of the resonance contributors shows the increased electron density of the ring, leading to increased rates of electrophilic substitution.[14]
Reactivity
editBecause of its partial aromatic character, furan's behavior is intermediate between that of an enol ether and an aromatic ring. It is dissimilar vs ethers such as tetrahydrofuran.
Like enol ethers, 2,5-disubstituted furans are susceptible to hydrolysis to reversibly give 1,4-diketones.
Furan serves as a diene in Diels–Alder reactions with electron-deficient dienophiles such as ethyl (E)-3-nitroacrylate.[15] The reaction product is a mixture of isomers with preference for the endo isomer:
Diels-Alder reaction of furan with arynes provides corresponding derivatives of dihydronaphthalenes, which are useful intermediates in synthesis of other polycyclic aromatic compounds.[16]
- It is considerably more reactive than benzene in electrophilic substitution reactions, due to the electron-donating effects of the oxygen heteroatom. It reacts with bromine at 0 °C to give 2-bromofuran.
- Hydrogenation of furans sequentially affords dihydrofurans and tetrahydrofurans.[citation needed]
- In the Achmatowicz reaction, furans are converted to dihydropyran compounds.
- Pyrrole can be prepared industrially by treating furan with ammonia in the presence of solid acid catalysts, such as SiO2 and Al2O3.[17]
Safety
editFuran is found in heat-treated commercial foods and is produced through thermal degradation of natural food constituents.[18][19] It can be found in roasted coffee, instant coffee, and processed baby foods.[19][20][21] Research has indicated that coffee made in espresso makers and coffee made from capsules contain more furan than that made in traditional drip coffee makers, although the levels are still within safe health limits.[22]
Exposure to furan at doses about 2,000 times the projected level of human exposure from foods increases the risk of hepatocellular tumors in rats and mice and bile duct tumors in rats.[23] Furan is therefore listed as a possible human carcinogen.[23]
See also
edit- BS 4994 – Furan resin as thermoset FRP for chemical process plant equipments
- Furanocoumarin
- Furanoflavonoid
- Furanose
- Furantetracarboxylic acid
- Simple aromatic rings
- Furan fatty acids
- Tetrahydrofuran
References
edit- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 392. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Jakubke, Hans Dieter; Jeschkeit, Hans (1994). Concise Encyclopedia of Chemistry. Walter de Gruyter. pp. 1–1201. ISBN 0-89925-457-8.
- ^ DHHS (NIOSH) Publication No. 2016–171, p. 2, Accessed Nov 2019
- ^ a b Hoydonckx, H. E.; Van Rhijn, W. M.; Van Rhijn, W.; De Vos, D. E.; Jacobs, P. A. "Furfural and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_119.pub2. ISBN 978-3527306732.
- ^ Senning, Alexander (2006). Elsevier's Dictionary of Chemoetymology. Elsevier. ISBN 0-444-52239-5.
- ^ Limpricht, H. (1870). "Ueber das Tetraphenol C4H4O". Berichte der Deutschen Chemischen Gesellschaft. 3 (1): 90–91. doi:10.1002/cber.18700030129.
- ^ Rodd, Ernest Harry (1971). Chemistry of Carbon Compounds: A Modern Comprehensive Treatise. Elsevier.
- ^ Wilson, W. C. (1941). "Furan". Organic Syntheses; Collected Volumes, vol. 1, p. 274.
- ^ Hou, X. L.; Cheung, H. Y.; Hon, T. Y.; Kwan, P. L.; Lo, T. H.; Tong, S. Y.; Wong, H. N. (1998). "Regioselective syntheses of substituted furans". Tetrahedron. 54 (10): 1955–2020. doi:10.1016/S0040-4020(97)10303-9.
- ^ a b Gilchrist, Thomas L. (1997). Heterocyclic Chemistry (3rd ed.). Liverpool: Longman. p. 209-212.
- ^ Adam Sniady, Marco S. Morreale, Roman Dembinski (2007). "Electrophilic Cyclization with N-Iodosuccinimide: Preparation of 5-(4-Bromophenyl)-3-Iodo-2-(4-Methyl-Phenyl)Furan". Organic Syntheses. 84: 199. doi:10.15227/orgsyn.084.0199.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ James A. Marshall, Clark A. Sehon (1999). "Isomerization of b-Alkynyl Allylic Alcohols to Furans Catalyzed by Silver Nitrate on Silica Gel: 2-Pentyl-3-Methyl-5-Heptylfuran". Organic Syntheses. 76: 263. doi:10.15227/orgsyn.076.0263.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 62, ISBN 978-0-471-72091-1
- ^ Bruice, Paula Y. (2007). Organic Chemistry (5th ed.). Upper Saddle River, NJ: Pearson Prentice Hall. ISBN 978-0-13-196316-0.
- ^ Masesane, I.; Batsanov, A.; Howard, J.; Modal, R.; Steel, P. (2006). "The oxanorbornene approach to 3-hydroxy, 3,4-dihydroxy and 3,4,5-trihydroxy derivatives of 2-aminocyclohexanecarboxylic acid". Beilstein Journal of Organic Chemistry. 2 (9): 9. doi:10.1186/1860-5397-2-9. PMC 1524792. PMID 16674802.
- ^ Filatov, M. A.; Baluschev, S.; Ilieva, I. Z.; Enkelmann, V.; Miteva, T.; Landfester, K.; Aleshchenkov, S. E.; Cheprakov, A. V. (2012). "Tetraaryltetraanthra[2,3]porphyrins: Synthesis, Structure, and Optical Properties" (PDF). J. Org. Chem. 77 (24): 11119–11131. doi:10.1021/jo302135q. PMID 23205621. Archived from the original (PDF) on 2020-02-19.
- ^ Harreus, Albrecht Ludwig. "Pyrrole". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_453. ISBN 978-3527306732.
- ^ Anese, M.; Manzocco, L.; Calligaris, S.; Nicoli, M. C. (2013). "Industrially Applicable Strategies for Mitigating Acrylamide, Furan and 5-Hydroxymethylfurfural in Food" (PDF). Journal of Agricultural and Food Chemistry. 61 (43): 10209–14. doi:10.1021/jf305085r. PMID 23627283. Archived from the original (PDF) on 2017-08-08.
- ^ a b Moro, S.; Chipman, J. K.; Wegener, J. W.; Hamberger, C.; Dekant, W.; Mally, A. (2012). "Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment" (PDF). Molecular Nutrition & Food Research. 56 (8): 1197–1211. doi:10.1002/mnfr.201200093. hdl:1871/41889. PMID 22641279. S2CID 12446132.
- ^ European Food Safety Authority (2011). "Update on furan levels in food from monitoring years 2004–2010 and exposure assessment". EFSA Journal. 9 (9): 2347. doi:10.2903/j.efsa.2011.2347.
- ^ Waizenegger, J.; Winkler, G.; Kuballa, T.; Ruge, W.; Kersting, M.; Alexy, U.; Lachenmeier, D. W. (2012). "Analysis and risk assessment of furan in coffee products targeted to adolescents". Food Additives & Contaminants: Part A. 29 (1): 19–28. doi:10.1080/19440049.2011.617012. PMID 22035212. S2CID 29027966.
- ^ "Espresso makers: Coffee in capsules contains more furan than the rest". Science Daily. April 14, 2011.
- ^ a b Bakhiya, N.; Appel, K. E. (2010). "Toxicity and carcinogenicity of furan in human diet" (PDF). Archives of Toxicology. 84 (7): 563–578. doi:10.1007/s00204-010-0531-y. PMID 20237914. S2CID 19389984.