Prochloraz, brand name Sportak, is an imidazole fungicide that was introduced in 1978[3] and is widely used in Europe, Australia, Asia, and South America within gardening and agriculture to control the growth of fungi.[4][5] It is not registered for use in the United States.[5] Similarly to other azole fungicides, prochloraz is an inhibitor of the enzyme lanosterol 14α-demethylase (CYP51A1), which is necessary for the production of ergosterol – an essential component of the fungal cell membrane – from lanosterol.[6] The agent is a broad-spectrum, protective and curative fungicide, effective against Alternaria spp., Botrytis spp., Erysiphe spp., Helminthosporium spp., Fusarium spp., Pseudocerosporella spp., Pyrenophora spp., Rhynchosporium spp., and Septoria spp.[5][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Abavit, Ascurit, Dibavit, Mirage, Octave, Omega, Prelude, Rival, Sporgon, Sportak, Sprint, Tenor[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.060.885 |
| Chemical and physical data | |
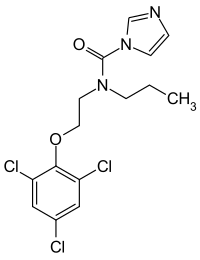
| Formula | C15H16Cl3N3O2 |
| Molar mass | 376.66 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Like many imidazole and triazole fungicides and antifungal medications, prochloraz is not particularly selective in its actions.[4][6] In addition to inhibition of lanosterol 14α-demethylase, prochloraz has also been found to act as an antagonist of the androgen and estrogen receptors, as an agonist of the aryl hydrocarbon receptor, and as an inhibitor of enzymes in the steroidogenesis pathway such as CYP17A1 and aromatase.[4][6] In accordance, it has been shown to produce reproductive malformations in mice.[4][6] As such, prochloraz is considered to be an endocrine disruptor.[4][6]
See also
editReferences
edit- ^ Anonymous AC05372279 (1997). Consolidated list of products whose consumption and/or sale have been banned, withdrawn, severely restricted or not approved by governments / Pharmaceuticals. United Nations Publications. pp. 576–. ISBN 978-92-1-130219-6.[permanent dead link]
- ^ a b Milne GW (2 September 2005). Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties. John Wiley & Sons. pp. 517–. ISBN 978-0-471-73661-5.
- ^ Carlile B (28 September 2006). "Chapter 3.7: Broad-spectrum systemic fungicides". Pesticide Selectivity, Health and the Environment. Cambridge University Press. pp. 81–. ISBN 978-1-139-45756-9.
- ^ a b c d e Vinggaard AM, Hass U, Dalgaard M, Andersen HR, Bonefeld-Jørgensen E, Christiansen S, et al. (February 2006). "Prochloraz: an imidazole fungicide with multiple mechanisms of action". International Journal of Andrology. 29 (1): 186–192. doi:10.1111/j.1365-2605.2005.00604.x. PMID 16466539.
- ^ a b c Paranjape K, Gowariker V, Krishnamurthy VN, Gowariker S (22 December 2014). "Prochloraz fungicide". The Pesticide Encyclopedia. CABI. pp. 406–. ISBN 978-1-78064-014-3.
- ^ a b c d e Sanderson JT (21 March 2015). "Disruptors of Androgen Action and Synthesis". In Darbre PD (ed.). Endocrine Disruption and Human Health. Elsevier Science. pp. 86–. ISBN 978-0-12-801120-1.
External links
edit- Prochloraz in the Pesticide Properties DataBase (PPDB)