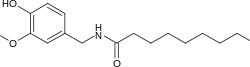
Nonivamide, also called pelargonic acid vanillylamide or PAVA, is an organic compound and a capsaicinoid. It is an amide of pelargonic acid (n-nonanoic acid) and vanillyl amine. It is present in chili peppers,[2] but is commonly manufactured synthetically. It is more heat-stable than capsaicin.
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-[(4-Hydroxy-3-methoxyphenyl)methyl]nonanamide | |
| Other names
Pseudocapsaicin; Vanillyl-N-nonylamide; Vanillylamide of n-nonanoic acid; VNA; Nonylic acid vanillyl amide; Pelargonic acid vanillylamide (PAVA); Pelargonyl vanillyl amide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.713 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H27NO3 | |
| Molar mass | 293.407 g·mol−1 |
| Appearance | White to off-white powder |
| Odor | Pungent |
| Density | 1.10 g/cm3 |
| Melting point | 54 °C (129 °F; 327 K) |
| Insoluble | |
| Solubility | Soluble in methanol |
| Hazards | |
| Flash point | 190 °C (374 °F; 463 K) (closed cup) |
| 330 °C (626 °F; 603 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
511 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Nonivamide | |
|---|---|
| Heat | Above peak |
| Scoville scale | 9,200,000[1] SHU |
Nonivamide is used as a food additive to add pungency to seasonings, flavorings, and spice blends. It is also used in the confectionery industry to create a hot sensation, and in the pharmaceutical industry in some formulations as a cheaper alternative to capsaicin.
Like capsaicin, it can deter mammals (but not birds or insects) from consuming plants or seeds (e.g. squirrels and bird feeder seeds).[3] This is consistent with nonivamide's role as a TRPV1 ion channel agonist. Mammalian TRPV1 is activated by heat and capsaicin, but the avian form is insensitive to capsaicin.[4]
Nonivamide is used (under the name PAVA) as the payload in "less-lethal munitions" such as the FN Herstal's FN 303 projectiles[5] or as the active ingredient in most pepper sprays,[3] which may be used as a chemical weapon.[6] As a chemical irritant, pepper sprays have been used both as a riot control munition and also a weapon to disperse peaceful demonstrators; they have also been used in other contexts, such as military or police training exercises.[6] While irritants commonly cause only "transient lacrimation, blepharospasm, superficial pain, and disorientation," their use and misuse also presents serious risks of more severe injury and disability.[6]
Treatment
editNonivamide is not soluble in water, however water will dilute it and wash it away. One study found that milk of magnesia, baby shampoo, 2% lidocaine gel, or milk, did not demonstrate significantly better performance than water, when used on pepper spray.[7]
See also
editReferences
edit- ^ Govindarajan, Sathyanarayana (1991). "Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences". Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
- ^ Howard L. Constant, Geoffrey A. Cordell and Dennis P. West (1996). "Nonivamide, a Constituent of Capsicum oleoresin". J. Nat. Prod. 59 (4): 425–426. doi:10.1021/np9600816.
- ^ a b http://www.aversiontech.com/hot-and-spicy/nonivamide-pava/Retrieved 16 July 2010 Archived 31 December 2015 at the Wayback Machine
- ^ Rohm, Barbara; Riedel, Annett; Ley, Jakob P; Widder, Sabine; Krammer, Gerhard E; Somoza, Veronika (2015). "Capsaicin, nonivamide and trans-pellitorine decrease free fatty acid uptake without TRPV1 activation and increase acetyl-coenzyme a synthetase activity in Caco-2 cells". Food & Function. 6 (1): 172–184. doi:10.1039/C4FO00435C. PMID 25422952.
- ^ "The FN 303 Less Lethal Launcher". Archived from the original on 2013-05-04. Retrieved 2013-04-14.
- ^ a b c Haar, Rohini J.; Iacopino, Vincent; Ranadive, Nikhil; Weiser, Sheri D.; Dandu, Madhavi (19 October 2017). "Health impacts of chemical irritants used for crowd control: a systematic review of the injuries and deaths caused by tear gas and pepper spray". BMC Public Health. 17 (1): 831. doi:10.1186/s12889-017-4814-6. PMC 5649076. PMID 29052530.
- ^ Barry, James D.; Hennessy, Robert; McManus, John G. (January 2008). "A Randomized Controlled Trial Comparing Treatment Regimens for Acute Pain for Topical Oleoresin Capsaicin (Pepper Spray) Exposure in Adult Volunteers". Prehospital Emergency Care. 12 (4): 432–437. doi:10.1080/10903120802290786. PMID 18924005. S2CID 12262260.