Vitamin B3, colloquially referred to as niacin, is a vitamin family that includes three forms, or vitamers: niacin (nicotinic acid), nicotinamide (niacinamide), and nicotinamide riboside.[1] All three forms of vitamin B3 are converted within the body to nicotinamide adenine dinucleotide (NAD).[1] NAD is required for human life and people are unable to make it within their bodies without either vitamin B3 or tryptophan.[1] Nicotinamide riboside was identified as a form of vitamin B3 in 2004.[2][1]
| Vitamin B3 | |
|---|---|
| Drug class | |
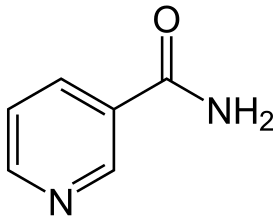 Structure of nicotinamide, one of the vitamers of vitamin B3 | |
| Class identifiers | |
| Use | Vitamin B3 deficiency |
| ATC code | A11H |
| Biological target | enzyme cofactor |
| Clinical data | |
| Drugs.com | Niacin |
| External links | |
| MeSH | D009536 |
| Legal status | |
| In Wikidata | |
Niacin (the nutrient) can be manufactured by plants and animals from the amino acid tryptophan.[3] Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds.[4][5] Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms of pellagra include skin and mouth lesions, anemia, headaches, and tiredness.[6] Many countries mandate its addition to wheat flour or other food grains, thereby reducing the risk of pellagra.[4][7]
The amide nicotinamide (niacinamide) is a component of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP+). Although niacin and nicotinamide are identical in their vitamin activity, nicotinamide does not have the same pharmacological, lipid-modifying effects or side effects as niacin, i.e., when niacin takes on the -amide group, it does not reduce cholesterol nor cause flushing.[8][9] Nicotinamide is recommended as a treatment for niacin deficiency because it can be administered in remedial amounts without causing the flushing, considered an adverse effect.[10] In the past, the group was loosely referred to as vitamin B3 complex.[11]
Mechanism of action
editNicotinamide adenine dinucleotide (NAD), along with its phosphorylated variant nicotinamide adenine dinucleotide phosphate (NADP), are utilized in transfer reactions within DNA repair and calcium mobilization. NAD also plays a critical role in human metabolism, acting as a coenzyme in both glycolysis and the Krebs cycle.[12]
Vitamin deficiency
editSevere vitamin B3 deficiency in the diet causes the disease pellagra, characterized by diarrhea, sun-sensitive dermatitis involving hyperpigmentation and thickening of the skin (see image), inflammation of the mouth and tongue, delirium, dementia, and if left untreated, death.[6] Common psychiatric symptoms include irritability, poor concentration, anxiety, fatigue, loss of memory, restlessness, apathy, and depression.[13] The biochemical mechanisms for the observed deficiency-caused neurodegeneration are not well understood, but may rest on A) the requirement for nicotinamide adenine dinucleotide (NAD+) to suppress the creation of neurotoxic tryptophan metabolites; B) inhibition of mitochondrial ATP generation resulting in cell damage; C) activation of the poly (ADP-ribose) polymerase (PARP) pathway, as PARP is a nuclear enzyme involved in DNA repair, but in the absence of NAD+ can lead to cell death; D) reduced synthesis of neuro-protective brain-derived neurotrophic factor or its receptor tropomyosin receptor kinase B; or, E) changes to genome expression directly due to the niacin deficiency.[14]
Niacin deficiency is rarely seen in developed countries, and it is more typically associated with poverty, malnutrition or malnutrition secondary to chronic alcoholism.[15] It also tends to occur in areas where people eat maize (corn) as a staple food, as maize is low in digestible niacin.[5] A cooking technique called nixtamalization, that is, pretreating with alkali ingredients, increases the bioavailability of niacin during maize meal or flour production.[16] For this reason, people who consume corn as tortillas or hominy are at less risk of niacin deficiency.
For treating deficiency, the World Health Organization (WHO) recommends administering niacinamide (i.e. nicotinamide) instead of niacin, to avoid the flushing side effect commonly caused by the latter. Guidelines suggest using 300 mg/day for three to four weeks.[10] Dementia and dermatitis show improvement within a week. Because deficiencies of other B-vitamins may be present, the WHO recommends a multi-vitamin in addition to the niacinamide.[10]
Hartnup disease is a hereditary nutritional disorder resulting in niacin deficiency.[17] It is named after an English family with a genetic disorder that resulted in a failure to absorb the essential amino acid tryptophan, tryptophan being a precursor for niacin synthesis. The symptoms are similar to pellagra, including red, scaly rash and sensitivity to sunlight. Oral niacin or niacinamide is given as a treatment for this condition in doses ranging from 50 to 100 mg twice a day, with a good prognosis if identified and treated early.[17] Niacin synthesis is also deficient in carcinoid syndrome, because of metabolic diversion of its precursor tryptophan to form serotonin.[4]
Measuring vitamin status
editPlasma concentrations of niacin and niacin metabolites are not useful markers of niacin status.[3] Urinary excretion of the methylated metabolite N1-methyl-nicotinamide is considered reliable and sensitive. The measurement requires a 24-hour urine collection. For adults, a value of less than 5.8 μmol/day represent deficient niacin status and 5.8 to 17.5 μmol/day represents low.[3] According to the World Health Organization, an alternative mean of expressing urinary N1-methyl-nicotinamide is as mg/g creatinine in a 24-hour urine collection, with deficient defined as <0.5, low 0.5-1.59, acceptable 1.6-4.29, and high >4.3[10] Niacin deficiency occurs before the signs and symptoms of pellagra appear.[3] Erythrocyte nicotinamide adenine dinucleotide (NAD) concentrations potentially provide another sensitive indicator of niacin depletion, although definitions of deficient, low and adequate have not been established. Lastly, plasma tryptophan decreases on a low niacin diet because tryptophan converts to niacin. However, low tryptophan could also be caused by a diet low in this essential amino acid, so it is not specific to confirming vitamin status.[3]
Dietary recommendations
edit
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The U.S. Institute of Medicine (renamed National Academy of Medicine in 2015) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for niacin in 1998, as well as Tolerable upper intake levels (ULs). In lieu of an RDA, Adequate Intakes (AIs) are identified for populations for which there is not enough evidence to identify a dietary intake level that is sufficient to meet the nutrient requirements of most people.[21] (see table).
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values (DRV), with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. For the EU, AIs and ULs have the same definition as in the US, except that units are milligrams per megajoule (MJ) of energy consumed rather than mg/day. For women (including those pregnant or lactating), men and children the PRI is 1.6 mg per megajoule. As the conversion is 1 MJ = 239 kcal, an adult consuming 2390 kilocalories should be consuming 16 mg niacin. This is comparable to US RDAs (14 mg/day for adult women, 16 mg/day for adult men).[22]
ULs are established by identifying amounts of vitamins and minerals that cause adverse effects, and then selecting as an upper limit amounts that are the "maximum daily intake unlikely to cause adverse health effects".[21] Regulatory agencies from different countries do not always agree. For the US, 30 or 35 mg for teenagers and adults, less for children.[3] The EFSA UL for adults is set at 10 mg/day – about one-third of the US value. For all of the government ULs, the term applies to niacin as a supplement consumed as one dose, and is intended as a limit to avoid the skin flush reaction. This explains why for EFSA, the recommended daily intake can be higher than the UL.[23]
Both the DRI and DRV describe amounts needed as niacin equivalents (NE), calculated as 1 mg NE = 1 mg niacin or 60 mg of the essential amino acid tryptophan. This is because the amino acid is utilized to synthesize the vitamin.[3][22]
For U.S. food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value (%DV). For niacin labeling purposes 100% of the Daily Value is 16 mg. Prior to May 27, 2016, it was 20 mg, revised to bring it into agreement with the RDA.[24][25] Compliance with the updated labeling regulations was required by January 1, 2020, for manufacturers with US$10 million or more in annual food sales, and by January 1, 2021, for manufacturers with lower volume food sales.[26][27] A table of the old and new adult daily values is provided at Reference Daily Intake.
Sources
editNiacin is found in a variety of whole and processed foods, including fortified packaged foods, meat from various animal sources, seafoods, and spices.[4][28] In general, animal-sourced foods provide about 5–10 mg niacin per serving, although dairy foods and eggs have little. Some plant-sourced foods such as nuts, legumes and grains provide about 2–5 mg niacin per serving, although in some grain products this naturally present niacin is largely bound to polysaccharides and glycopeptides, making it only about 30% bioavailable. Fortified food ingredients such as wheat flour have niacin added, which is bioavailable.[5] Among whole food sources with the highest niacin content per 100 grams:
| Source[29] | Amount (mg / 100g) |
|---|---|
| Nutritional yeast[30] Serving = 2 Tbsp (16 g) contains 56 mg |
350 |
| Tuna, yellowfin | 22.1 |
| Peanuts | 14.3 |
| Peanut butter | 13.1 |
| Bacon | 10.4 |
| Tuna, light, canned | 10.1 |
| Salmon | 10.0 |
| Turkey depending on what part, how cooked | 7-12 |
| Chicken depending on what part, how cooked | 7-12 |
| Source[29] | Amount (mg / 100g) |
|---|---|
| Beef depending on what part, how cooked | 4-8 |
| Pork depending on what part, how cooked | 4-8 |
| Sunflower seeds | 7.0 |
| Tuna, white, canned | 5.8 |
| Almonds | 3.6 |
| Mushrooms, white | 3.6 |
| Cod fish | 2.5 |
| Rice, brown | 2.5 |
| Hot dogs | 2.0 |
| Source[29] | Amount (mg / 100g) |
|---|---|
| Avocado | 1.7 |
| Potato, baked, with skin | 1.4 |
| Corn (maize) | 1.0 |
| Rice, white | 0.5 |
| Kale | 0.4 |
| Eggs | 0.1 |
| Milk | 0.1 |
| Cheese | 0.1 |
| Tofu | 0.1 |
Vegetarian and vegan diets can provide adequate amounts if products such as nutritional yeast, peanuts, peanut butter, tahini, brown rice, mushrooms, avocado and sunflower seeds are included. Fortified foods and dietary supplements can also be consumed to ensure adequate intake.[5][31]
Food preparation
editNiacin naturally found in food is susceptible to destruction from high heat cooking, especially in the presence of acidic foods and sauces. It is soluble in water, and so may also be lost from foods boiled in water.[32]
Food fortification
editCountries fortify foods with nutrients to address known deficiencies.[7] As of 2020, 54 countries required food fortification of wheat flour with niacin or niacinamide; 14 also mandate fortification of maize flour, and 6 mandate fortification of rice.[33] From country to country, niacin fortification ranges from 1.3 to 6.0 mg/100 g.[33]
As a dietary supplement
editIn the United States, niacin (the acid) is sold as a non-prescription dietary supplement with a range of 100 to 1000 mg per serving. These products often have a Structure/Function health claim[34] allowed by the US Food & Drug Administration (FDA). An example would be "Supports a healthy blood lipid profile." The American Heart Association (AHA) strongly advises against the use of non-prescription dietary supplement niacin rather than prescription niacin because of potentially serious side effects. For this reason and because the manufacture of dietary supplement niacin is not as well-regulated by the FDA as is prescription niacin, the AHA advises that supplemental niacin only be used under the supervision of a health care professional.[35] More than 30 mg niacin consumed as a dietary supplement can cause skin flushing. Face, arms and chest skin turns a reddish color because of vasodilation of small subcutaneous blood vessels, accompanied by sensations of heat, tingling and itching. These signs and symptoms are typically transient, lasting minutes to hours; they are considered unpleasant rather than toxic.[5]
Toxicity
editThe daily limit for vitamin B3 has been set at 35 mg. At daily doses of as low as 30 mg, flushing has been reported, always starting in the face and sometimes accompanied by skin dryness, itching, paresthesia, and headache.[12] Liver toxicity is the most serious toxic reaction and it occurs at doses >2 grams/day.[36] Fulminant hepatitis has been reported at doses between 3-9 grams/day with needs for liver transplantation.[citation needed] Other reactions include glucose intolerance, hyperuricemia, macular edema, and macular cysts.[12]
History
editCorn (maize) became a staple food in the southeast United States and in parts of Europe. A disease that was characterized by dermatitis of sunlight-exposed skin was described in Spain in 1735 by Gaspar Casal. He attributed the cause to poor diet.[37] In northern Italy it was named pellagra from the Lombard language (agra = holly-like or serum-like; pell = skin).[38][39] In time, the disease was more closely linked specifically to corn.[40] In the US, Joseph Goldberger was assigned to study pellagra by the Surgeon General of the United States. His studies confirmed a corn-based diet as the culprit, but he did not identify the root cause.[41][42]
Nicotinic acid was extracted from the liver by biochemist Conrad Elvehjem in 1937. He later identified the active ingredient, referring to it as "pellagra-preventing factor" and the "anti-blacktongue factor."[43] It was also referred to as "vitamin PP", "vitamin P-P" and "PP-factor", all derived from the term "pellagra-preventive factor".[10] In the late 1930s, studies by Tom Douglas Spies, Marion Blankenhorn, and Clark Cooper confirmed that niacin cured pellagra in humans. The prevalence of the disease was greatly reduced as a result.[44]
In 1942, when flour enrichment with nicotinic acid began, a headline in the popular press said "Tobacco in Your Bread." In response, the Council on Foods and Nutrition of the American Medical Association approved of the Food and Nutrition Board's new names niacin and niacin amide for use primarily by non-scientists. It was thought appropriate to choose a name to dissociate nicotinic acid from nicotine, to avoid the perception that vitamins or niacin-rich foods contain nicotine, or that cigarettes contain vitamins. The resulting name niacin was derived from nicotinic acid + vitamin.[45][46]
J. Laguna and K.J. Carpenter found in 1951, that niacin in corn is biologically unavailable and can be released only in very alkaline lime water of pH 11. This explains why a Latin-American culture that used alkali-treated (nixtamalized) cornmeal to make tortilla was not at risk for niacin deficiency.[47]
References
edit- ^ a b c d Stipanuk MH, Caudill MA (2013). Biochemical, Physiological, and Molecular Aspects of Human Nutrition - E-Book. Elsevier Health Sciences. p. 541. ISBN 9780323266956.
Vitamin B3... potentially includes three different molecular forms: nicotinic acid, niacinamide, and nicotinamide riboside
- ^ Bieganowski P, Brenner C (May 14, 2004). "Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans". Cell. 117 (4): 495–502. doi:10.1016/s0092-8674(04)00416-7. PMID 15137942.
- ^ a b c d e f g h i j Institute of Medicine (1998). "Niacin". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 123–149. ISBN 9780309065542. Retrieved August 29, 2018.
- ^ a b c d "Niacin". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. October 8, 2018. Retrieved September 16, 2019.
- ^ a b c d e "Niacin Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. June 3, 2020. Retrieved June 29, 2020.
- ^ a b Hegyi J, Schwartz RA, Hegyi V (January 2004). "Pellagra: dermatitis, dementia, and diarrhea". International Journal of Dermatology. 43 (1): 1–5. doi:10.1111/j.1365-4632.2004.01959.x. PMID 14693013. S2CID 33877664.
- ^ a b "Why fortify?". Food Fortification Initiative. 2017. Archived from the original on April 4, 2017. Retrieved April 4, 2017.
- ^ Jaconello P (October 1992). "Niacin versus niacinamide". CMAJ. 147 (7): 990. PMC 1336277. PMID 1393911.
- ^ Kirkland JB (May 2012). "Niacin requirements for genomic stability". Mutation Research. 733 (1–2): 14–20. doi:10.1016/j.mrfmmm.2011.11.008. PMID 22138132.
- ^ a b c d e World Health Organization (2000). Pellagra And Its Prevention And Control In Major Emergencies (Report). World Health Organization (WHO). hdl:10665/66704. WHO/NHD/00.10.
- ^ Silvestre R, Torrado E (2018). Metabolic Interaction in Infection. Springer. p. 364. ISBN 9783319749327.
Niacin or nicotinate, together with its amide form nicotinamide, defines the group of vitamin B3 complex
- ^ a b c Suter P, Russell R (2018). "Chapter 326: Vitamin and Trace Mineral Deficiency and Excess". Harrison's Principles of Internal Medicine, 20th Ed. New York: McGraw-Hill. ISBN 9781259644030.
- ^ Penberthy W, Kirkland J (2020). "Niacin". In Marriott B, Birt D, Stallings V, Yates A (eds.). Present Knowledge in Nutrition (11 ed.). London, United Kingdom: Academic Press (Elsevier). pp. 209–224. ISBN 9780323661621.
- ^ Fu L, Doreswamy V, Prakash R (August 2014). "The biochemical pathways of central nervous system neural degeneration in niacin deficiency". Neural Regeneration Research. 9 (16): 1509–13. doi:10.4103/1673-5374.139475. PMC 4192966. PMID 25317166.
- ^ Pitsavas S, Andreou C, Bascialla F, Bozikas VP, Karavatos A (March 2004). "Pellagra encephalopathy following B-complex vitamin treatment without niacin". International Journal of Psychiatry in Medicine. 34 (1): 91–5. doi:10.2190/29XV-1GG1-U17K-RGJH. PMID 15242145. S2CID 29070525. Archived from the original on July 10, 2012. Retrieved November 27, 2009.
- ^ Bressani R, Gomez-Brenes R, Scrimshaw NS (1961). "Effect of processing on distribution and in vitro availability of niacin of corn (Zea mays)". Food Technol. 15: 450–4.
- ^ a b LaRosa CJ (January 2020). "Hartnup Disease". Archived from the original on July 8, 2020. Retrieved July 6, 2020.
- ^ a b "Nutrient reference values for Australia and New Zealand" (PDF). National Health and Medical Research Council. September 9, 2005. Archived from the original (PDF) on January 21, 2017. Retrieved June 19, 2018.
- ^ a b Health Canada (July 20, 2005). "Dietary Reference Intakes". Government of Canada. Retrieved June 20, 2018.
- ^ a b c "Tolerable Upper Intake Levels for Vitamins and Minerals" (PDF). European Food Safety Authority. February 2006. Retrieved June 18, 2018.
- ^ a b "Nutrient Recommendations: Dietary Reference Intakes (DRI)". National Institutes of Health, Office of Dietary Supplements. Retrieved June 30, 2020.
- ^ a b "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017.
- ^ "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006.
- ^ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels" (PDF).
- ^ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on April 7, 2020. Retrieved May 16, 2020.
- ^ "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). May 27, 2016. Retrieved May 16, 2020. This article incorporates text from this source, which is in the public domain.
- ^ "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). December 21, 2018. Retrieved May 16, 2020. This article incorporates text from this source, which is in the public domain.
- ^ "Niacin content per 100 grams; select food subset, abridged list by food groups". United States Department of Agriculture, Agricultural Research Service, USDA Branded Food Products Database v.3.6.4.1. January 17, 2017. Archived from the original on February 2, 2017. Retrieved January 23, 2017.
- ^ a b c "USDA National Nutrient Database for Standard Reference Legacy: Niacin" (PDF). U.S. Department of Agriculture, Agricultural Research Service. 2018. Retrieved May 12, 2020.
- ^ "Nutritional Yeast Flakes (two tablespoons = 16 grams". NutritionData.Self.com. Retrieved May 13, 2020.
- ^ "Vitamin B3 (Niacin)". VivaHealth.org. 2000. Archived from the original on August 4, 2020. Retrieved May 12, 2020.
- ^ "Effects of Cooking on Vitamins (Table)". Beyondveg. Archived from the original on October 16, 2012. Retrieved April 30, 2019.
- ^ a b "Map: Count of Nutrients In Fortification Standards". Global Fortification Data Exchange. Retrieved July 4, 2020.
- ^ "Structure/Function Claims". U.S. Food & Drug Administration. December 2017. Retrieved June 30, 2020.
- ^ "Cholesterol Medications". American Heart Association. November 10, 2018. Retrieved May 8, 2020.
- ^ Goodman LS, Hardman JG, Limbird LE, Gilman AG (2001). Goodman & Gilman's the pharmacological basis of therapeutics (10 ed.). New York: McGraw-Hill. ISBN 0071354697. OCLC 46548349.
- ^ Casal G (1945). "The natural and medical history of the principality of the Asturias". In Major RH (ed.). Classic Descriptions of Disease (3 ed.). Springfield: Charles C Thomas. pp. 607–12.
- ^ F. Cherubini, Vocabolario Milanese-Italiano, Imp. Regia Stamperia, 1840–43, vol. I, III.
- ^ "Definition of Pellagra". MedicineNet.com. Archived from the original on September 30, 2007. Retrieved June 18, 2007.
- ^ Cesare Lombroso, Studi clinici ed esperimentali sulla natura, causa e terapia delle pellagra (Bologna: Fava e Garagnani, 1869)
- ^ Evans BK, Feinstein AR (September 1994). "Joseph Goldberger: an unsung hero of American clinical epidemiology". Ann Intern Med. 121 (5): 372–75. doi:10.7326/0003-4819-121-5-199409010-00010. PMID 8042827. S2CID 13226008.
- ^ Kraut A. "Dr. Joseph Goldberger and the War on Pellagra | Ashes on the Potomac". history.nih.gov. Retrieved February 20, 2017.
- ^ Elvehjem CA, Madden RJ, Strongandd FM, Woolley DW (1938). "The isolation and identification of the anti-blacktongue factor J" (PDF). J. Biol. Chem. 123 (1): 137–49. doi:10.1016/S0021-9258(18)74164-1.
- ^ Ruth Hanna Sachs, White Rose History. Volume I. 2003. Appendix D, p. 2 ISBN 978-0-9710541-9-6 "Men of the Year, outstanding in comprehensive science were three medical researchers who discovered that nicotinic acid was a cure for human pellagra: Drs. Tom Douglas Spies of Cincinnati General Hospital, Marion Arthur Blankenhorn of the University of Cincinnati, Clark Niel Cooper of Waterloo, Iowa."
- ^ "Niacin and Niacin Amide". Journal of the American Medical Association. 118 (10): 819. March 7, 1942. doi:10.1001/jama.1942.02830100049011.
- ^ "Niacin and Nicotinic Acid". Journal of the American Medical Association. 118 (10): 823. March 7, 1942. doi:10.1001/jama.1942.02830100053014.
- ^ Laguna J, Carpenter KJ (September 1951). "Raw versus processed corn in niacin-deficient diets". The Journal of Nutrition. 45 (1): 21–8. doi:10.1093/jn/45.1.21. PMID 14880960.