 | |
| Clinical data | |
|---|---|
| Trade names | Dovprela |
| Other names | Pretomanid FGK, PA-824 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619056 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Nitroimidazole[1] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
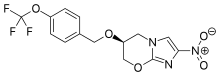
| Formula | C14H12F3N3O5 |
| Molar mass | 359.261 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pretomanid, sold under the brand name Dovprela, is an antibiotic used to treat multi-drug-resistant tuberculosis affecting the lungs.[4] It is generally used together with bedaquiline and linezolid.[3] It is taken by mouth.[3]
Common side effects include nerve damage, acne, vomiting, headache, low blood sugar, diarrhea, and liver inflammation.[4] Other side effects may include bone marrow suppression, optic neuropathy, and QT prolongation.[5] Safety in pregnancy is unclear.[6] It is in the nitroimidazole class of medications.[1]
Pretomanid was approved for medical use in the United States in 2019 and Europe in 2020.[4][3] It is on the World Health Organization's List of Essential Medicines.[7] In the developing world it cost 364 USD for 6 months in 2019.[8] In the United States this amount costs about 3,800 USD as of 2021.[9] It was developed by the TB Alliance.[1][4]
References edit
- ^ a b c "Our Pipeline". TB Alliance. Archived from the original on 7 April 2019. Retrieved 18 April 2019.
- ^ "Pretomanid tablet". DailyMed. 15 September 2019. Archived from the original on 21 October 2021. Retrieved 25 September 2020.
- ^ a b c d e f "Pretomanid FGK EPAR". European Medicines Agency (EMA). 24 March 2020. Archived from the original on 20 October 2020. Retrieved 25 September 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c d e "FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs". U.S. Food and Drug Administration (FDA) (Press release). 14 August 2019. Archived from the original on 19 August 2019. Retrieved 28 August 2019. This article incorporates text from this source, which is in the public domain.
- ^ "Pretomanid Monograph for Professionals". Drugs.com. Retrieved 29 October 2021.
- ^ "Pretomanid Use During Pregnancy". Drugs.com. Retrieved 29 October 2021.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Price announced for new lifesaving TB drug pretomanid still too high". Médecins Sans Frontières Access Campaign. Archived from the original on 3 March 2021. Retrieved 29 October 2021.
- ^ "Pretomanid Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 January 2021. Retrieved 29 October 2021.