 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | (/ˌsaɪproʊˈhɛptədiːn/[1] |
| Trade names | Periactin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682541 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | First-generation antihistamine[2][3] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 96 to 99% |
| Metabolism | Liver[5][6] Mostly CYP3A4 mediated. |
| Elimination half-life | 8.6 hours[4] |
| Excretion | Faecal (2-20%; 34% of this as unchanged drug) and renal (40%; none as unchanged drug)[5][6] |
| Identifiers | |
| |
| Chemical and physical data | |
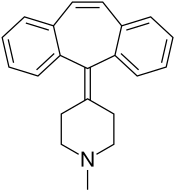
| Formula | C21H21N |
| Molar mass | 287.406 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cyproheptadine, sold under the brand name Periactin among others, is a first-generation antihistamine primarily used to treat allergies.[2][3] This may include itchiness, hay fever, and hives.[3] It is taken by mouth.[2]
Common side effects include sleepiness, dizziness, agitation, and poor coordination.[2] Other side effects may include swelling, problems urinating, and increased weight.[3] There is no evidence of harm with use during pregnancy, however such use has not been well studied.[7] Care should be taken in those at risk of glaucoma.[3]
Cyproheptadine was patented in 1959 and came into medical use in 1961.[8] It is available as a generic medication.[9] In the United Kingdom 30 tablets of 4 mg costs the NHS about 6 pounds in 2020.[3] This amount in the United States costs about 10 USD.[9]
References edit
- ^ "Cyproheptadine". Dictionary.com Unabridged (Online). n.d.
- ^ a b c d e f "Cyproheptadine Hydrochloride Monograph for Professionals". Drugs.com. Archived from the original on 11 December 2018. Retrieved 19 November 2020.
- ^ a b c d e f g BNF 79. London: Pharmaceutical Press. March 2020. p. 292. ISBN 978-0857113658.
- ^ Gunja N, Collins M, Graudins A (2004). "A comparison of the pharmacokinetics of oral and sublingual cyproheptadine". Journal of Toxicology. Clinical Toxicology. 42 (1): 79–83. doi:10.1081/clt-120028749. PMID 15083941. S2CID 20196551.
- ^ a b "CYPROHEPTADINE HYDROCHLORIDE tablet [Boscogen, Inc.]" (PDF). DailyMed. Boscogen, Inc. November 2010. Archived from the original on 4 July 2013. Retrieved 26 October 2013.
- ^ a b "PRODUCT INFORMATION PERIACTIN® (cyproheptadine hydrochloride)" (PDF). Aspen Pharmacare Australia. Aspen Pharmacare Australia Pty Ltd. 17 November 2011. Archived from the original (PDF) on 29 October 2013. Retrieved 26 October 2013.
- ^ "Cyproheptadine (Periactin) Use During Pregnancy". Drugs.com. Archived from the original on 25 November 2020. Retrieved 19 November 2020.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 547. ISBN 9783527607495. Archived from the original on 2021-08-29. Retrieved 2020-09-19.
- ^ a b "Cyproheptadine". Archived from the original on 11 November 2016. Retrieved 19 November 2020.