 | |
| Clinical data | |
|---|---|
| Trade names | Bretaris Genuair, Eklira Genuair, Tudorza Pressair |
| Other names | Aclidinium bromide |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Inhalation |
| Drug class | Long-acting muscarinic antagonist (LAMA)[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <5% (in system) 30% (in lung) |
| Metabolism | Ester hydrolysis |
| Elimination half-life | 2–3 hrs |
| Duration of action | >24 hrs |
| Excretion | 65% urine, 33% faeces |
| Identifiers | |
| |
| Chemical and physical data | |
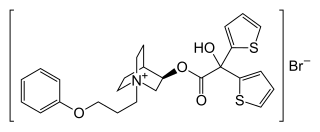
| Formula | C26H30BrNO4S2 |
| Molar mass | 564.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Aclidinium is a medication used for maintenance treatment of chronic obstructive pulmonary disease (COPD).[1] It may improve quality of life and prevent hospitalization; but does not affect the risk of death or the need for steroids.[2] It is unclear if it differs from tiotropium or other medications in the LAMA class.[2] It is used by a dry powder inhaler.[1]
Common side effects include headache, cough, and inflammation of the nose and throat.[1] Other side effects may include diarrhea, bronchospasm, and urinary retention.[3][1] It is a long-acting muscarinic antagonist (LAMA).[1]
Aclidinium was approved for medical use in the United States in 2012.[1] In the United Kingdom a month costs the NHS about 33 pounds.[3] In the United States this amount costs about 560 USD as of 2021.[4] It is also available together with formoterol.[3]
References edit
- ^ a b c d e f g h i "Aclidinium Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 15 July 2021.
- ^ a b Ni, H; Soe, Z; Moe, S (2014). "Aclidinium bromide for stable chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (9): CD010509. doi:10.1002/14651858.CD010509.pub2. PMID 25234126.
- ^ a b c BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 260. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Aclidinium Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 15 July 2021.