A hypervalent molecule is a molecule that contains one or more typical elements (group 1, 2, 13-18) formally bearing more than eight electrons in their valence shells. Phosphorus pentachloride (PCl5), sulfur hexafluoride (SF6), the phosphate (PO43−) ion, chlorine trifluoride (ClF3) and the triiodide (I3−) ion are examples of hypervalent molecules.
Definitions and nomenclature edit
Hypervalent molecules were first formally defined by Jeremy I. Musher in 1969 as molecules having central atoms of group 15-18 in any oxidation state other than the lowest.[1]
Several specific classes of hypervalent molecules exist:
- Hypervalent iodine compounds are useful reagents in organic chemistry (ex. the Dess-Martin periodinane)
- Tetra-, penta- and hexacoordinated phosphorous, silicon, and sulfur compounds (ex. PCl5, PF5, SF6, sulfuranes and persulfuranes)
- Noble gas compounds (ex. xenon tetrafluoride, XeF4)
- Halogen polyfluorides (ex. ClF5)
- Non-classical carbocations (ex. Norbornyl cation)[2]
- Many common acids (ex. chloric acid, phosphorous acid, and sulfuric acid)
N-X-L notation edit
N-X-L nomenclature, introduced in 1980,[3] is often used to classify hypervalent compounds of main group elements, where:
- N represents the number of valence electrons
- X is the chemical symbol of the central atom
- L the number of ligands to the central atom
Examples of N-X-L nomenclature include:
History and controversy edit
The debate over the nature and classification of hypervalent molecules goes back to Gilbert Lewis and Irving Langmuir and the debate over the the nature of the chemical bond in the 1920s.[4] Lewis maintained the importance of the two-center two-electron (2c-2e) bond in describing hypervalence, thus allowing for expanded octets. Langmuir, on the other hand, upheld the dominance of the octet rule and preferred the use of ionic bonds to account for hypervalence without violating it (e.g. SF42+, F22−).
In the late 1920s and 1930s, Sugden argued for the existence of a two-center one-electron (2c-1e) bond and thus rationalized bonding in hypervalent molecules without the need for expanded octets or ionic bond character; this was poorly accepted at the time.[4] In the 1940s and 1950s, Rundle and Pimentel popularized the idea of the three-center four-electron bond, which is essentially the same concept which Sugden attempted to advance decades earlier; the three-center four-electron bond can be alternatively viewed as consisting of two collinear two-center one-electron bonds, with the remaining two nonbonding electrons localized to the ligands.[4]
The attempt to actually prepare hypervalent organic molecules began with Hermann Staudinger and Georg Wittig in the first half of the twentieth century, who sought to challenge the extant valence theory and successfully prepare nitrogen and phosphorous-centered hypervalent molecules.[5] The theoretical basis for hypervalency was not delineated until J.I. Musher's work in 1969.[1].
In 1990, Magnusson published a seminal work definitively excluding the role of d-orbital hybridization in bonding in hypervalent compounds of second-row elements. This had long been a point of contention and confusion in describing these molecules using molecular orbital theory. Part of the confusion here originates from the fact that one must include d-functions in the basis sets used to describe these compounds (or else unreasonably high energies and distorted geometries result), and the contribution of the d-function to the molecular wavefunction is large. These facts were historically interpreted to mean that d-orbitals must be involved in bonding. However, Magnusson concludes in his work that d-orbital involvement is not implicated in hypervalency.[6]
Both the term and concept of hypervalency still fall under criticism. In 1984, in response to this general controversy, Paul von Ragué Schleyer proposed the replacement of 'hypervalency' with use of the term hypercoordination because this term does not imply any mode of chemical bonding and the question could thus be avoided altogether.[4]
The concept itself has been criticized by Ronald Gillespie who, based on an analysis of electron localization functions, wrote in 2002 that "as there is no fundamental difference between the bonds in hypervalent and non-hypervalent (Lewis octet) molecules there is no reason to continue to use the term hypervalent."[7]
Bonding in hypervalent molecules edit
Early considerations of the structure of hypervalent molecules, returned familiar arrangements that were well explained by the VSEPR model for atomic bonding. Accordingly, AB5 and AB6 type molecules would possess a trigonal bi-pyramidal and octahedral geometry, respectively. However in order to account for the observed bond angles, bond lengths and apparent violation of the Lewis octet rule, several alternative models have been proposed [8].
In the 1950s molecular orbital treatment of hypervalent bonding was adduced to explain the molecular architecture. According to MO theory, the central atom of penta- and hexacoordinated molecules would be sp3d and sp3d2 hybridized, which requires the promotion of central atom electrons to unoccupied d-orbitals. However, advances in the study of ab initio calculations have revealed that the contribution of d-orbitals to hypervalent bonding is too small to describe the bonding properties, and this hybrid orbital description is now regarded as much less important [9]. It was shown that in the case of hexacoordinated SF6, d-orbitals are not involved in S-F bond formation, but charge transfer between the sulfur and fluorine atoms and the apposite resonance structures were able to account for the hypervalency.
Additional modifications to the octet rule have been attempted to involve ionic characteristics in hypervalent bonding. As one of these modifications, in 1951, the concept of the 3-center-4-electron (3c-4e) bond, which described hypervalent bonding with a qualitative molecular orbital, was proposed[10]. The 3c-4e bond is described as three molecular orbitals given by the combination of a p orbital on the central atom and two ligand orbitals leading to an occupied non-bonding orbital (HOMO), and an unoccupied anti-bonding orbital (LUMO). This model in which the octet rule is preserved was also advocated by Musher.[4].

An example of this is the hexacoordinated SF6, which has been proposed to be composed of three 3c-4e bonds. In this model each bond is equivalent, linear and orthogonal with one lying along each of x, y and z axes. These interactions are F(p1)-S(3px2)-F(p1), F(p1)-S(3py2)-F(p1), and F(p1)-S(3pz2)-F(p1). Together these data account for both the octahedral symmetry of the molecule as well as observed molecular structure
A more complete description of hypervalent molecules arises from consideration of bonding as a whole-molecule phenomenon through quantum mechanical methods. A LCAO in, for example, sulfur hexafluoride, taking a basis set of the three sulfur p-orbitals and eighteen fluorine p-orbitals, a total of twenty four molecular orbitals are obtained, providing room for all 48 electrons in bonding, nonbonding, and antibonding MOs.
Structure, reactivity, and kinetics edit
Structure edit
Pentacoordinated phosphorous edit
For hypervalent compounds in which the ligands are more electronegative than the central, hypervalent atom, resonance structures can be drawn with no more than four covalent electron pair bonds and completed with ionic bonds to obey the octet rule. For example, phosphorous pentafluoride’s three equatorial bonds can be formed from sp2-hybridized phosphorus orbitals. The axial bonds can then be described by two resonance forms each containing one ionic bond and one covalent bond, thus satisfying the octet rule and explaining both the observed molecular geometry and relative discrepancy between the axial and equatorial bond lengths. The axial bonds may be represented as two half-bonds (the ‘average’ of the symmetrical resonance forms) or a single 3c-4e bond. However, the magnitude of the discrepancy between the axial and equatorial bond lengths is substantially smaller than this structural model predicts. [11]

Hexacoordinated sulfur edit
For a hexacoordinate molecule such as sulfur hexafluoride, each of the six bonds is the same length. The rationalization described above can be applied to generate resonance structures each with two covalent bonds and two 3c-4e bonds, such that the 3c-4e bond character is distributed across each of the sulfur-fluorine bonds.

Hexacoordinated phosphorous edit
Hexacoordinate phosphorous molecules involving nitrogen, oxygen, or sulfur ligands provide examples of Lewis acid-Lewis base hexacoordination.[12] For the two similar complexes shown below, the length of the C-P bond increases with decreasing length of the N-P bond; the strength of the C-P bond decreases with increasing strength of the N-P Lewis acid-Lewis base interaction.
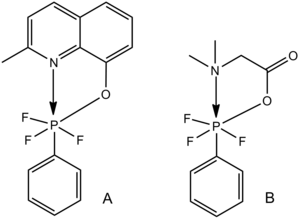
Pentacoordinated silicon edit
This trend is also generally true of pentacoordinated main-group elements with one or more lone-pair-containing ligand, including the oxygen-pentacoordinated silicon examples shown below.

Interestingly, complexes such as these provide a model for the SN2 transition state; the Si-O bonds range from close to the expected van der Waals value in A (a weak bond, representing an early SN2 transition state) almost to the expected covalent single bond value in C (a strong bond, representing a late SN2 transition state).[12]
Reactivity edit
Silicon edit
Corriu and coworkers performed early work characterizing reactions thought to proceed through a hypervalent transition state [13]. Measurements of the rates of hydrolysis of tetravalent chlorosilanes incubated with catalytic amounts of water returned a rate that is first order in chlorosilane and second order in water. This indicated that two water molecules interacted with the silane during hydrolysis and from this a binucleophilic reaction mechanism was proposed. Corriu and coworkers then measured the rates of hydrolysis in the presence of nucleophilic catalyst HMPT, DMSO or DMF. It was shown that the rate of hydrolysis was again first order in chlorosilane, first order in catalyst and now first order in water. Appropriately, the rates of hydrolysis also exhibited a dependence on the magnitude of charge on the oxygen of the nucleophile.
Taken together this led the group to propose a reaction mechanism in which there is a pre-rate determining nucleophilic attack of the tetracoordinated silane by the nucleophile (or water) in which a hypervalent pentacoordinated silane is formed. This is followed by a nucleophilic attack of the intermediate by water in a rate determining step leading to hexacoordinated species that quickly decomposes giving the hydroxysilane.
Silane hydrolysis was further investigated by Holmes and coworkers [14] in which tetracoordinated Mes2SiF2 (Mes = mesityl) and pentacoordinated Mes2SiF3- were reacted with two equivalents of water. Following twenty-four hours, almost no hydrolysis of the tetracoordinated silane was observed, while the pentacoordinated silane was completely hydrolyzed after fifteen minutes. Additionally, X-ray diffraction data collected for the tetraethylammonium salts of the fluorosilanes showed the formation of hydrogen bisilonate lattice supporting a hexacoordinated intermediate from which HF2- is quickly displaced leading to the hydroxylated product. This reaction and crystallographic data support the mechanism proposed by Corriu et al.


The apparent increased reactivity of hypervalent molecules, contrasted with tetravalent analogues, has also been observed for Grignard reactions. The Corriu group measured [15] Grignard reaction half-times by NMR for related 18-crown-6 potassium salts of a variety of tetra- and pentacoordinated methylphenylfluorosilanes in the presence of catalytic amounts of nucleophile.
Though the half reaction method is imprecise, the magnitudinal differences in reactions rates allowed for a proposed reaction scheme wherein, a pre-rate determining attack of the tetravalent silane by the nucleophile results in an equilibrium between the neutral tetracoordinated species and the anionic pentavalent compound. This is followed by nucleophilic coordination by two Grignard reagents as normally seen, forming a hexacoordinated transition state and yielding the expected product.

Phosphorous edit
Similar reactivity has also been observed for other hypervalent structures such as the miscellany of phosphorous compounds, for which hexacoordinated transition states have been proposed. Hydrolysis of phosphoranes and oxyphosphoranes have been studied [16]and shown to be second order in water. Bel'skii et al. have proposed a prerate determining nucleophilic attack by water resulting in an equilibrium between the penta- and hexacoordinated phosphorous species, which is followed by a proton transfer involving the second water molecule in a rate determining ring-opening step, leading to the hydroxlyated product.

Alchoholysis of pentacoordinated phosphorous compounds, such as trimethoxyphospholene with benzyl alcohol, have also been postulated to occur through a similar octahedral transition state, as in hydrolysis, however without ring opening [17].

It can be understood from these experiments that the increased reactivity observed for hypervalent molecules, contrasted with analogous nonhypervalent compounds, can be attributed to the congruence of these species to the hypercoordinated activated states normally formed during the course of the reaction.
Ab initio calculations edit
The enhanced reactivity at pentacoordinated silicon is not fully understood. Corriu and coworkers suggested that greater electropositive character at the pentavalent silicon atom may be responsible for its increased reactivity. [18] Preliminary ab initio calculations supported this hypothesis to some degree, but used a small basis set.[19]
A software program for ab initio calculations, Gaussian 86, was used by Dieters and coworkers to compare tetracoordinated silicon and phosphorus to their pentacoordinate analogues. This ab initio approach is used as a supplement to determine why reactivity improves in nucleophilic reactions with pentacoordinated compounds. For silicon, the 6-31+G* basis set was used because of its pentacoordinated anionic character and for phosphorus, the 6-31G* basis set was used. [19]
Pentacoordinated compounds should theoretically be less electrophilic than tetracoordinated analogues due to steric hindrance and greater electron density from the ligands, yet experimentally show greater reactivity with nucleophiles than their tetracoordinated analogues. Advanced ab initio calculations were performed on series of tetracoordinated and pentacoordinated species to further understand this reactivity phenomenon. Each series varied by degree of fluorination. Bond lengths and charge densities are shown as functions of how many hydride ligands are on the central atoms. For every new hydride, there is one less fluoride. [19]
For silicon and phosphorus bond lengths, charge densities, and Mulliken bond overlap, populations were calculated for tetra and pentacoordinated species by this ab initio approach. [19]Addition of a fluoride ion to tetracoordinated silicon shows an overall average increase of 0.1 electron charge, which is considered insignificant. In general, bond lengths in trigonal bipyramidal pentacoordinate species are longer than those in tetracoordinate analogues. Si-F bonds and Si-H bonds both increase in length upon pentacoordination and related effects are seen in phosphorus species, but to a lesser degree. The reason for the greater magnitude in bond length change for silicon species over phosphorus species is the increased effective nuclear charge at phosphorus. Therefore, silicon is concluded to be more loosely bound to its ligands.
In addition Dieters and coworkers [19] show an inverse correlation between bond length and bond overlap for all series. Pentacoordinated species are concluded to be more reactive because of their looser bonds as trigonal-bipyramidal structures.
The energies for addition and removal of a fluoride ion in silicon and phosphorus species were calculated.

As the table shows, tetracoordinated species have much higher energy requirements for ligand removal than do pentacoordinated species. Overall, silicon species have lower energy requirements for ligand removal than do phosphorus species, which is an indication of weaker bonds in silicon.
In conclusion, it has been shown that charge density changes are insignificant in accounting for enhanced reactivity with nucleophiles in hypercoordinated silicon and phosphorus. On the other hand, enhanced reactivity is due to weaker bonds, particularly in the axial positions, of pentacoordinated species. [19] \
Application edit
The mechanistic implications of this are extended to a hexacoordinated silicon species, which is thought to be active as a transition state in reactions such as the allylation of aldehydes with allyltrifluorosilane. The reaction only precedes with fluoride activation to the pentacoordinated state and weakening of the bond between silicon and carbon in the hexacoordinate state drives this reaction. [20]

See also edit
References edit
- ^ a b Musher, J.I. (1969). "The Chemistry of Hypervalent Molecules". Angew. Chem. Int. 8: 54–68. doi:10.1002/anie.196900541.
- ^ Eric Anslyn; Dennis Dougherty. Modern Physical Organic Chemistry. University Science Books. ISBN 9781891389313.
{{cite book}}: Unknown parameter|city=ignored (|location=suggested) (help) - ^ Perkins,C. W.; Martin, J. C.; Arduengo, A. J.; Lau, W.; Alegria, A,; Kochi, J. K.; An Electrically Neutral a-Sulfuranyl Radical from the Homolysis of a Perester with Neighboring Sulfenyl Sulfur: 9-S-3 species J.Am. Chem. Soc. 1980, 102, 7753-7759 doi:10.1021/ja00546a019
- ^ a b c d e Jensen, W. (2006). "The Origin of the Term "Hypervalent"". J. Chem. Ed. 83: 1751. | Link
- ^ Kin-ya Akiba. Chemistry of Hypervalent Compounds. Wiley VCH. ISBN 0471240192.
{{cite book}}: Unknown parameter|city=ignored (|location=suggested) (help) - ^ E. Magnusson. Hypercoordinate molecules of second-row elements: d functions or d orbitals? J. Am. Chem. Soc. 1990, 112, 7940-7951. doi:10.1021/ja00178a014
- ^ Gillespie, R. J.; Silvi, B. The octet rule and hypervalence: two misunderstood concepts. Coord. Chem. Rev. 2002, 233-234, 53-62. [1]
- ^ Xiaoping Sun (2002). Chem. Educator. 7: 11–14. doi:10.1007/s00897010525a.
{{cite journal}}: Missing or empty|title=(help) - ^ E. Magnusson. Hypercoordinate molecules of second-row elements: d functions or d orbitals? J. Am. Chem. Soc. 1990, 112, 7940-7951. doi:10.1021/ja00178a014
- ^ Xiaoping Sun (2002). Chem. Educator. 7: 261–264. doi:10.1007/s00897020598a.
{{cite journal}}: Missing or empty|title=(help) - ^ Gillespie, R.J.; Silvi, B. (2002). "The Octet Rule and Hypervalence: Two Misunderstood Concepts". Coordination Chemistry Reviews. 53: 233–234. doi:10.1016/S0010-8545(02)00102-9.
- ^ a b c d Holmes, R.R. (1996). "Comparison of Phosphorus and Silicon: Hypervalency, Stereochemistry, and Reactivity". Chem. Rev. 96: 927–950. doi:10.1021/cr950243n.
- ^ Corriu, RJP (1978). J. Organomet. Chem. 150: 27–38.
{{cite journal}}: Missing or empty|title=(help) - ^ Johnson, SE; Deiters, JA; Day, RO; Holmes, RR (1989). J. Am. Chem. Soc. 111: 3250.
{{cite journal}}: Missing or empty|title=(help) - ^ Corriu, RJP (1988). Organometallics. 7: 237–8.
{{cite journal}}: Missing or empty|title=(help) - ^ Bel'Skii, VE (1979). J. Gen. Chem. USSR. 49: 298.
{{cite journal}}: Missing or empty|title=(help) - ^ Ramirez, F (1968). J. Am.Chem. Soc. 90: 751.
{{cite journal}}: Missing or empty|title=(help) - ^ Corriu, R.; Guerin, C.; Henner, B.; Wong Chi Man, W.W.C (1988). "Pentacoordinated silicon anions: reactivity toward strong nucleophiles". Organometallics. 7: 7197–7202. doi:10.1021/ja00176a018.
- ^ a b c d e f Dieters, J. A.; Holmes, R. R. (1990). "Enhanced Reactivity of Pentacoordinated Silicon Species. An ab Initio Approach". J. Am. Chem. Soc. 112: 7197–7202. doi:10.1021/ja00176a018. Cite error: The named reference "abinitio1" was defined multiple times with different content (see the help page).
- ^ Kira, M; Kobayashi, M.; Sakurai, H. (1987). "Regiospecific and highly stereoselective allylation of aldehydes with allyltrifluorosilane activated by fluoride ions". Tetrahedron Letters. 28: 4081–4084. doi:10.1016/S0040-4039(01)83867-3.

