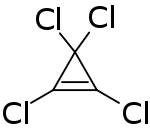
Tetrachlorocyclopropene is a chemical compound with the formula C3Cl4. A colorless liquid, the compound is a reagent used to prepare acetylene derivatives and in organic synthesis.[1] It is prepared by addition of dichlorocarbene to trichloroethylene.[2] It can react with water and alcohols rapidly.[3]
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.025.835 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3Cl4 | |
| Molar mass | 177.83 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.45 g/mL |
| Boiling point | 125 - 130 C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The compound is used to prepare arylpropiolic acids:
- C3Cl4 + ArH + 2 H2O → ArC2CO2H + 4 HCl
Under some circumstances, diarylation occurs, giving diarylcyclopropenones, which decarbonylate to give diarylacetylenes. These reactions are thought to proceed via the intermediacy of trichlorocyclopropenium electrophile (C3Cl3+).
References edit
- ^ Oliver Reiser; Armin de Meijere (2001). "Tetrachlorocyclopropene". EEROS. doi:10.1002/047084289X.rt028. ISBN 0-471-93623-5.
- ^ Glück, C; Poingée, V; Schwager, H (1987). "Improved Synthesis of 7,7-Difluorocyclopropabenzene". Synthesis. 1987 (3): 260–262. doi:10.1055/s-1987-27908. S2CID 96607067.
- ^ Pentachlorocyclopropane Stephen W. Tobey and Robert West. The University of Wisconsin (1965)