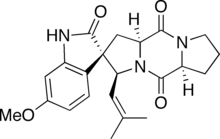
Spirotryprostatin A is an indolic alkaloid from the 2,5-Diketopiperazine class of natural products found in the Aspergillus fumigatus fungus. Spirotryprostatin A and several other indolic alkaloids (including Spirotryprostatin B, as well as other tryprostatins and cyclotryprostatins) have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs.[1] Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2S,3S,5aS,10aS)-2′-Hydroxy-6′-methoxy-3-(2-methylprop-1-en-1-yl)-1,5a,6,7,8,10,10a-hexahydro-3H,5H,10H-spiro[dipyrrolo[1,2-a:1′,2′-d]pyrazine-2,3′-indole]-5,10-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C22H25N3O4 | |
| Molar mass | 395.43 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
One such total synthesis was published in 1999, which showed that the isopropylidene side chain was not necessary to the biological activity of the compound, leading to a number of new theorized analogues.[2]
References edit
- ^ Borthwick AD (2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews. 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- ^ Edmondson, S; et al. (1999). "Total Synthesis of Spirotryprostatin A, Leading to the Discovery of Some Biologically Promising Analogues". J. Am. Chem. Soc. 121 (10): 2147–2155. doi:10.1021/ja983788i.