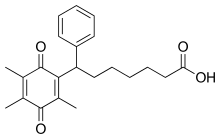
Seratrodast (development name, AA-2414; marketed originally as Bronica)[2] is a thromboxane A2 (TXA2) receptor (TP receptor) antagonist used primarily in the treatment of asthma.[3][4] It was the first TP receptor antagonist that was developed as an anti-asthmatic drug and received marketing approval in Japan in 1997.[5] As of 2017 seratrodast was marketed as Bronica in Japan, and as Changnuo, Mai Xu Jia, Quan Kang Nuo in China.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Bronica in Japan, Changnuo, Mai Xu Jia, Quan Kang Nuo in China and as Seradair in India. .[1] |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets, granules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >96% |
| Elimination half-life | 22 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.176 |
| Chemical and physical data | |
| Formula | C22H26O4 |
| Molar mass | 354.446 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Unlike thromboxane synthase inhibitors such as ozagrel, seratrodast does not affect thrombus formation, time to occlusion and bleeding time.[6] Seratrodast has no effect on prothrombin time and activated partial thromboplastin time, thus ruling out any action on blood coagulation cascade.[7]
Medical uses edit
Seratrodast is used to treat asthma.[8][9]
There are no adequate and well-controlled studies of seratrodast in pregnant women. The drug should be used in pregnancy only if the potential benefits justify the risk to the fetus.[9] Seratrodast should not be used during lactation.[9]
The safety and efficacy of seratrodast has not been established in children (<18 years of age).[9]
Contraindications and interactions edit
Seratrodast should not be used in people with liver disease.[9]
Use with paracetamol or with cephem antibiotics increases the risk of liver damage. Use with aspirin increases the bioavailability of seratrodast.[9]
Adverse effects edit
The most frequently observed (0.1 to 5%) adverse reactions include elevated transaminases, nausea, loss of appetite, stomach discomfort, abdominal pain, diarrhea, constipation, dry mouth, taste disturbance, drowsiness, headache, dizziness, palpitations and malaise.[9] Less than 0.1% of patients experienced vomiting, thrombocytopenia, epistaxis, bleeding tendency, insomnia, tremor, numbness, hot flushes and edema.[9] All the adverse reactions reported were of mild to moderate severity, and resolved when the drug was discontinued.[9]
Pharmacology edit
Thromboxane A2 (TXA2) is generated in the lungs of people with asthma, and when it signals through the thromboxane receptor it causes bronchoconstriction, vasoconstriction, mucous secretion, and airway hyper-responsiveness. Seratrodast inhibits the activity of the thromboxane receptor, blocking the effects of TXA2.[10]
Pharmacokinetics edit
The pharmacokinetics of seratrodast have been studied in Japanese and Caucasian, including Indian, healthy volunteers.[11][12][13][14] The plasma concentrations of seratrodast increase with increasing doses. The absorption of seratrodast is relatively rapid with maximum plasma concentrations of 4.6–6 μg/ml obtained in 3 to 4 hours.[11] Steady state plasma concentrations of seratrodast are reached within 4–5 days.[13] Seratrodast is slowly cleared, mainly by hepatic biotransformation. The drug shows biexponential decay in plasma profiles with a mean elimination half-life of 22 hours.[11][13] Approximately 20% of the administered dose is recovered in the urine, with 60% of the urinary recovery being in the form of conjugates [12]
Chemistry edit
Seratrodast can be prepared in five steps starting from pimelic acid monoester.[15]
History edit
Seratrodast was the first thromboxane receptor antagonist to reach the market as a treatment for asthma; it was approved in Japan in 1997.[8]
Society and culture edit
As of 2017 seratrodast was marketed as Bronica in Japan, Changnuo, Mai Xu Jia, Quan Kang Nuo in China and as Seretra & Seradair in India.[1]
Research edit
Seratrodast was studied in perennial allergic rhinitis, chronic bronchitis and chronic pulmonary emphysema but efforts to bring the drug to market in those indications was abandoned around 2000.[2]
References edit
- ^ a b c "Seratrodast international brands". Drugs.com. Retrieved 8 March 2017.
- ^ a b "Seratrodast". AdisInsight. Retrieved 8 March 2017.
- ^ Endo S, Akiyama K (November 1996). "[Thromboxane A2 receptor antagonist in asthma therapy]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 54 (11): 3045–8. PMID 8950952.
- ^ Hada S, Hashizume M, Nishii S, Yoshioka F, Yasunaga K (January 1993). "[Study on the inhibitory effect of AA-2414 on platelet aggregation and its clinical effect in asthmatic patients]". Arerugi [Allergy] (in Japanese). 42 (1): 18–25. PMID 8457165.
- ^ Dogné JM, de Leval X, Benoit P, Delarge J, Masereel B (2002). "Thromboxane A2 inhibition: therapeutic potential in bronchial asthma". American Journal of Respiratory Medicine. 1 (1): 11–7. doi:10.1007/bf03257158. PMID 14720071. S2CID 40324562.
- ^ Dogné JM, Hanson J, de Leval X, Kolh P, Tchana-Sato V, de Leval L, et al. (May 2004). "Pharmacological characterization of N-tert-butyl-N'-[2-(4'-methylphenylamino)-5-nitrobenzenesulfonyl]urea (BM-573), a novel thromboxane A2 receptor antagonist and thromboxane synthase inhibitor in a rat model of arterial thrombosis and its effects on bleeding time". The Journal of Pharmacology and Experimental Therapeutics. 309 (2): 498–505. doi:10.1124/jpet.103.063610. PMID 14742735. S2CID 46723447.
- ^ Samara EE (1996). "Seratrodast (AA-2414)—A Novel Thromboxane-A2 Receptor Antagonist". Cardiovascular Drug Reviews. 14 (3): 272–85. doi:10.1111/j.1527-3466.1996.tb00231.x.
- ^ a b Rolin S, Masereel B, Dogné JM (March 2006). "Prostanoids as pharmacological targets in COPD and asthma". European Journal of Pharmacology. 533 (1–3): 89–100. doi:10.1016/j.ejphar.2005.12.058. PMID 16458293.
- ^ a b c d e f g h i "医療用医薬品 : ブロニカ (Japanese label)" (in Japanese). KEGG. October 2016. Retrieved 8 March 2017.
- ^ Dogné JM, de Leval X, Benoit P, Rolin S, Pirotte B, Masereel B (February 2002). "Therapeutic potential of thromboxane inhibitors in asthma". Expert Opinion on Investigational Drugs. 11 (2): 275–81. doi:10.1517/13543784.11.2.275. PMID 11829716. S2CID 19276801.
- ^ a b c An open-labeled, randomized, cross-over bioequivalence study of Seratrodast 80mg under fasting condition. Data on file (appears on website on Seretra)
- ^ a b Hiraga K, Tateno M (1993). "The clinical phase I study of AA-2414, a thromboxane A, receptor antagonist – repeated-dose study at 160 mg once daily for 7 days". Clin Pharmacol. 9 (Suppl. 8): 41–55.
- ^ a b c Hussein Z, Samara E, Locke CS, Orchard MA, Ringham GL, Granneman GR (April 1994). "Characterization of the pharmacokinetics and pharmacodynamics of a new oral thromboxane A2-receptor antagonist AA-2414 in normal subjects: population analysis". Clinical Pharmacology and Therapeutics. 55 (4): 441–50. doi:10.1038/clpt.1994.54. PMID 8162671. S2CID 20801213.
- ^ Samara EE, Qian J, Locke C, Dean R, Killian A, Granneman GR (1996). "Single-dose and steady-state pharmacokinetics of seratrodast in healthy male and female volunteers". Pharm Res. 13 (Suppl. 9).
- ^ Shiraishi M, Kato K, Terao S, Ashida Y, Terashita Z, Kito G (September 1989). "Quinones. 4. Novel eicosanoid antagonists: synthesis and pharmacological evaluation". Journal of Medicinal Chemistry. 32 (9): 2214–21. doi:10.1021/jm00129a030. PMID 2769691.