Osedax is a genus of deep-sea siboglinid polychaetes, commonly called boneworms, zombie worms, or bone-eating worms. Osedax is Latin for "bone-eater". The name alludes to how the worms bore into the bones of whale carcasses to reach enclosed lipids, on which they rely for sustenance. They utilize specialized root tissues for bone-boring. It is possible that multiple species of Osedax reside in the same bone.[2] Osedax worms are also known to feed on the collagen itself by making holes in the whale's skeletal structure. These holes can also serve as a form of protection from nearby predators.
| Osedax Temporal range:
| |
|---|---|
| |
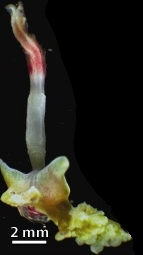
| Osedax roseus | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Annelida |
| Clade: | Pleistoannelida |
| Clade: | Sedentaria |
| Order: | Sabellida |
| Family: | Siboglinidae |
| Genus: | Osedax Rouse et al., 2004[1] |
| Species | |
|
See text. | |
Scientists from the Monterey Bay Aquarium Research Institute using the submarine ROV Tiburon first discovered the genus in Monterey Bay, California, in February 2002. The worms were found living on the bones of a decaying gray whale in the Monterey Canyon, at a depth of 2,893 m (9,491 ft).
Anatomy and physiology edit
Osedax are colorful tubeworms that have no mouth, anus, or gut.[3] The body is divided into different regions: trunk, ovisac, and root. They range in length between 2.5 to 7 cm (1 to 3 inches), although this varies between species (cite).[4] Sexual dimorphism is observed in Osedax with females >20,000 times larger than males.[5]
Digestive System edit
Osedax rely on symbiotic species of bacteria that aid in the digestion of whale proteins and lipids and release nutrients that the worms can absorb.[6] Osedax have colorful feathery plumes that also act as gills and unusual root-like structures that absorb nutrients. The Osedax secrete acid (rather than rely on teeth) to bore into bone to access the nutrients.[7] High concentrations of carbonic anhydrase are found in the roots of Osedax. This serves as evidence of a common bioerosion mechanism in which secreted acid is produced by anaerobic respiration. This process works with a demineralization mechanism in which oxygen is carried from seawater to the roots and HCO3- is secreted into the seawater.[8]
The epidermis also plays key roles in bone deterioration and nutrient uptake. This process of bone deterioration occurs through a symbiotic relationship with an endosymbiotic bacteria.[9] The cells in the epidermis of the Osedax root region are responsible for the secretion of digestive enzymes. The epidermis also has an expanded microvillus border which increases the surface area.[9]
Through the use of X-ray CT technology, scans showed that borings made by Osedax mucofloris were hemi-ellipsoidal in shape. Boring depths varied depending on which bone was colonized by the O. mucofloris. Deeper borings were found in radius bone compared to the ulna and vertebrae.[10]
Osedax roots are covered by a mucus sheath that helps protect the worm's trunk. Some studies support the theory that this sheath plays a role in dissolving the bone. This sheath could also play an important role in reducing the damage to Osedax skin by absorbing harmful acid. Another potential function of the mucus sheath is that it could inhibit the breakdown of the worm's bone matrix. This is significant because the bone matrix is integral in maintaining the worm's position while in direct contact with a bone.[8]
Sexual dimorphism edit
Osedax males are notably smaller than their female counterparts. Between 50 and 100 microscopic dwarf males live inside the tube surrounding a single female and never develop past the larval stage and produce sperm.[5] Male dwarfism prevents competition with female Osedax worms for food and space.[5] Conditions that favour dwarfism in male Osedax are:
- Eliminates competition between male and female Osedax as resources are limited,[11]
- Sessile lifestyle: attach to and rely on females for food,[11]
- Decreases difficulty in finding a mate.[11]
Interestingly, Osedax priapus lack the frequently observed sexual size dimorphism, and males have similar size to females. This results in competition for space and food.[5] These male worms are able to produce more sperm. However, sexual size dimorphism is still observed in O. priapus where most males are one-third the volume of females.
Reproduction edit
Discoveries edit
Female Osedax worms have been observed spawning both in the wild and in laboratory aquaria.[12] Osedax rubiplumus can spawn hundreds of oocytes at a time. They are already fertilized when they are released from the female worm. The worms' endosymbionts, species of bacteria in the order Oceanospirillales, were not observed in the spawned oocytes, which suggests that they are acquired after the worms settle on the bones.[12] In the adult, the bacteria are localised in the root-like structures that grow into the whale bone.[13][14] This worm appears to be highly fecund and reproduces continuously. This may help explain why Osedax is such a diverse genus, despite the rarity of whale falls in the ocean.
Male Osedax are microscopic dwarfs that live as "harems" inside the lumen of the gelatinous tube that surrounds each female. An individual female can house hundreds of these males in her tube.[15][16]
Following its discovery in 2002 by researchers at the Monterey Bay Aquarium Research Institute, the genus was announced in Science in 2004.[1]
In late 2005, an experiment by Swedish marine biologists resulted in the discovery of a species of the worm in the North Sea off the west coast of Sweden. In the experiment, a minke whale carcass that had been washed ashore had been sunk to a depth of 120 m (390 ft) and monitored for several months. Biologists were surprised to find that, unlike the previous discoveries, the new species, colloquially known as "bone-eating snot flower" after its scientific name (Osedax mucofloris), lived in relatively shallow waters.
In November 2009, researchers reported finding as many as 15 species of boneworms living in Monterey Bay on the California coast.[17]
Sex Determination edit
Annelid sex is typically determined by genetic factors,[18] however models of environmental sex determination have been proposed for Osedax, in which larvae that settle on bones mature into females, while larvae that settle on female Osedax do not fully develop and mature into males.[19] O. japonicus in particular has showcased an environmental form of sex determination.[20]
Life Cycle[21] edit
- Mature female Osedax worms spawn eggs into the mucus attached to their tubes, where the embryos develop for 3 days.
- Larvae then begin to swim in the water column. This is called the trochophore stage. The larvae settle on whale bones and begin crawling.
- During the trocophore stage, male Osedax settle on the tubes of the females, where they are metamorphosed into dwarf males, which can be inside or outside the female tube.
- 1 day after settling on bones, larvae use two pairs of chaetae to attach to the substrate. Juvenile worms begin to secrete mucus and develop two ventral palps on the dorsal side of the prostomium.
- 2 days after settling, the palps elongate and the heart starts to beat. The roots attach to the bones begin to digest.
- 4 days after settling, the trunk and ventral palps elongate, where symbiotic bacteria are detected in the root.
- 7 days after settlement, pinnules extend from the ventral palps.
- 10 days post settlement, the juvenile worms have 4 palps with pinnules, an oviduct, and a distinct root system.
Symbiosis edit
Symbionts are the primary providers of nutrition for Osedax.[22] However, these symbionts also possess genes, secretion systems, and toxins that disrupt the Osedax membrane and facilitate recurrent infections of adult Osedax through the root tips.[22][23] There is ongoing debate in the literature over whether the symbiosis in Osedax roots is commensal or mutualistic.[24][23] The symbiotic relationship between Osedax and its accompanying bacteria may be transferred either via vertical or horizontal transmission.[23]
Osedax species use collagen, which is the primary organic component in bone.[25] Collagen is degraded using a family of endopeptidases called matrix metalloproteinases (MMPs), which facilitates nutrient absorption by the Osedax.[25] The roots of the Osedax express high amounts of V-ATPase and carbonic anhydrase enzymes, which allows the Osedax to dissolve and absorb collagen and lipids.[25] Once dissolved, the nutrients are either used by the Osedax, or transported to the symbionts for further catabolism.[23][25]
As the endosymbionts lack secreted M9 peptidase, they rely on the Osedax worm to source extracellular collagen.[25] The symbionts in the Oceanospirillales order have then been observed to further process the collagen using collagenolytic enzymes.[23][26]
Sequencing of the Osedax worm genome has suggested an evolved dependency on its endosymbionts.[25] This is revealed by genomic streamlining, where increased functional groups were observed despite the loss of some gene families.[25] Six incomplete pathways were discovered in the Osedax worm genome which were supplemented by the endosymbionts.[25] In particular, the Osedax worm lacks specific gene families involved in bone lipid and carbohydrate metabolism.[25] This function is complemented by the Oceanospirillales symbionts, which utilize the glyoxylate cycle to catabolize nutrients from whale bones and convert fatty acids into carbohydrates.[25] The Osedax are then able to take up and store the end products as glycogen.[25] Bacteriocytes are present in the Osedax lower trunk subepidermal connective tissue,[25] and there are additional genes in the bacteriocytes that encode amino acids and glucose and aid in digestion and absorption of proteins into the roots.[27]
Endosymbionts edit
The Oceanospirillales symbionts are found in the specialized roots[24] of all Osedax species,[28][23] and play a major role in accelerating the degradation process of bones, as well as facilitating nutrient uptake for the Osedax.[24][25] Oceanospirillales are known for their ability to degrade complex organic compounds.[22][26]
Campylobacterales are abundant along the trunk of the Osedax according to a 2023 study.[24] Different genera in this order are found in Osedax at different points during the whale’s degradation:
- Members of the Arcobacter genus are the primary early colonizers (<24 months).[24]
- Sulfurospirillum genus members colonize at ~50 months, during the transitional stages of organic carbon breakdown.[24]
- The Sulfurimonas genus dominates at >140 months, and are key players in its symbiosis with the Osedax host.[24]
The Sulfurimonas genus in particular protects the Osedax worms from potentially harmful by-products produced at >140 months of the whale fall degradation.[24] The Sulfurimonas bacteria house the type II and IV sulfide:quinone oxidoreductase genes that encode enzymes to oxidize and assimilate sulfide.[24] These reactions prevent the host from absorbing toxic by-products across the epithelial barrier.
Niche edit
The role of Osedax in the degradation of marine vertebrate remains controversial. Some scientists[29] think that Osedax is a specialist on whalebones while others think that it is more of a generalist.[30][31] This controversy is due to a biogeographic paradox: despite the rarity and ephemeral nature of whale falls, Osedax has a broad biogeographic range and is surprisingly diverse. One hypothesis advanced to explain this paradox is that Osedax are able to colonize a variety of vertebrate remains besides whalebones. This hypothesis is supported by an experiment involving cow bones suspended above the sea floor. A variety of Osedax species successfully colonized these bones. Osedax have also been observed colonizing terrestrial mammal bones mixed in with galley waste from a surface vessel. Other scientists have countered this hypothesis by pointing out how the cow bone experiment does not match any natural habitat and also the low probability of terrestrial mammal bones arriving at the ocean floor in significant quantities. They also point out other cases of food falls in which the remains disappeared too swiftly for Osedax colonization and the lack of any observed colonization in similar cases. The true role of Osedax in the degradation of marine vertebrate remains is important to marine vertebrate taphonomy. Burrows closely similar to those made by Osedax species have been found in the bones of ancient marine birds and plesiosaurs, suggesting that the genus may once have had a wider range of foods.[32][33][34] In a study of the boring morphological diversity of Osedax, it was shown that the species difference of bone-boring is highly variable; within the same species, the boring morphology is only consistent in a particular bone, but not consistent in different bones. It was also suggested that multiple species of Osedax can co-exist in the same bone and in an incomplete spatial niche differentiation.[2]
The function of Osedax and their borings welcome other species such as Stephonyx amphipods, Paralomis crabs, and Rubyspira gastropods. As Osedax worms break down bone and lipid layers, fauna take advantage and colonize these bone matrices. Overall, the borings made by Osedax have shown to enhance biodiversity and the worms should, therefore, be considered ecosystem engineers. The downside of the deterioration caused by Osedax is that it speeds up the process of erosion, therefore only allowing this new fauna their new habitats for a temporary period.[35]
Evolution edit
The oldest trace fossils on bones characteristic of Osedax are from a plesiosaur humerus from the Cambridge Greensand, England, likely reworked from late Albian (~100 million years old) sediments and a rib and costal plate from a sea turtle found in Cenomanian (100-93 million years ago) aged sediments of the Chalk Group, England. Osedax likely persisted on the bones of sea turtles after the extinction of most large marine reptiles at the end of the Cretaceous.[36] Osedax have the generalist ability to feed on different vertebrates (fishes, marine birds, whale bones).[37]
In terms of evolutionary history research, the Osedax could have had negative impact in preserving fossil record because its appearance at the shelf-depth combined with its ability to efficiently break down marine vertebrates skeletons.[36]
Species edit
Selected species:[38][39][40][41]
- Osedax antarcticus Glover, Wiklund & Dahlgren, 2013
- Osedax braziliensis Fujiwara, Jimi, Sumida, Kawato, Kitazato
- Osedax bryani Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax crouchi Amon, Wiklund, Dahlgren, Copley, Smith, Jamieson & Glover, 2014
- Osedax deceptionensis Taboada, Cristobo, Avila, Wiklund & Glover, 2013
- Osedax docricketts Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax frankpressi Rouse, Goffredi & Vrijenhoek, 2004
- Osedax jabba Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax japonicus Fujikura, Fujiwara & Kawato, 2006
- Osedax knutei Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax lehmani Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax lonnyi Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax mucofloris Glover, Kallstrom, Smith & Dahlgren, 2005
- Osedax nordenskjoeldi Amon, Wiklund, Dahlgren, Copley, Smith, Jamieson & Glover, 2014
- Osedax priapus Rouse et al., 2014
- Osedax packardorum Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax randyi Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax rogersi Amon, Wiklund, Dahlgren, Copley, Smith, Jamieson & Glover, 2014
- Osedax roseus Rouse, Worsaae, Johnson, Jones & Vrijenhoek, 2008
- Osedax rubiplumus Rouse, Goffredi & Vrijenhoek, 2004
- Osedax ryderi Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax sigridae Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax talkovici Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax tiburon Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax ventana Rouse, Goffredi, Johnson & Vrijenhoek
- Osedax westernflyer Rouse, Goffredi, Johnson & Vrijenhoek
References edit
- ^ a b G. W. Rouse; S. K. Goffredi & R. C. Vrijenhoek (2004). "Osedax: Bone-Eating Marine Worms with Dwarf Males". Science. 305 (5684): 668–671. Bibcode:2004Sci...305..668R. doi:10.1126/science.1098650. PMID 15286372. S2CID 34883310.
- ^ a b Higgs, Nicholas D.; Glover, Adrian G.; Dahlgren, Thomas G.; Smith, Craig R.; Fujiwara, Yoshihiro; Pradillon, Florence; Johnson, Shannon B.; Vrijenhoek, Robert C.; Little, Crispin T. S. (2014). "The morphological diversity of Osedax worm borings (Annelida: Siboglinidae)" (PDF). Journal of the Marine Biological Association of the United Kingdom. 94 (7): 1429–1439. Bibcode:2014JMBUK..94.1429H. doi:10.1017/S0025315414000770. S2CID 52246559.
- ^ Lazaro, Enrico de (July 5, 2012). "Study Finds Osedax Worms Use Bone-Melting Acid | Biology | Sci-News.com". Sci.News: Breaking Science News. Retrieved April 9, 2024.
- ^ Admin, L. L. S. (February 2, 2022). "Osedax : The Whale-Bone-Eating Zombie Worm". Learn Life Science. Retrieved April 9, 2024.
- ^ a b c d Rouse, Greg W.; Wilson, Nerida G.; Worsaae, Katrine; Vrijenhoek, Robert C. (January 19, 2015). "A Dwarf Male Reversal in Bone-Eating Worms". Current Biology. 25 (2): 236–241. Bibcode:2015CBio...25..236R. doi:10.1016/j.cub.2014.11.032. ISSN 0960-9822. PMID 25496962.
- ^ Marlow, Jeffrey (February 18, 2019). "A Whale's Afterlife". The New Yorker. ISSN 0028-792X. Retrieved February 20, 2019.
- ^ "Bone-eating 'zombie' worms drill with acid". BBC News.
- ^ a b Tresguerres, Martin; Katz, Sigrid; Rouse, Greg W. (June 22, 2013). "How to get into bones: proton pump and carbonic anhydrase in Osedax boneworms". Proceedings of the Royal Society B: Biological Sciences. 280 (1761): 20130625. doi:10.1098/rspb.2013.0625. PMC 3652447. PMID 23760644.
- ^ a b Katz, Sigrid; Klepal, Waltraud; Bright, Monika (October 2010). "The skin of Osedax (Siboglinidae, Annelida): An ultrastructural investigation of its epidermis". Journal of Morphology. 271 (10): 1272–1280. doi:10.1002/jmor.10873. PMID 20672365. S2CID 10697873.
- ^ Higgs, Nicholas D.; Glover, Adrian G.; Dahlgren, Thomas G.; Little, Crispin T. S. (December 2011). "Bone-Boring Worms: Characterizing the Morphology, Rate, and Method of Bioerosion by Osedax mucofloris (Annelida, Siboglinidae)". The Biological Bulletin. 221 (3): 307–316. doi:10.1086/bblv221n3p307. ISSN 0006-3185. PMID 22186919. S2CID 32725146.
- ^ a b c Trivers, Robert L. (November 8, 1974). "Sexuality: Costs and Benefits: The Economy of Nature and the Evolution of Sex . Michael T. Ghiselin. University of California Press,. Berkeley,. 1974. xii, 346 pp. $12.95". Science. 186 (4163): 525–526. doi:10.1126/science.186.4163.525. ISSN 0036-8075.
- ^ a b G. W. Rouse; N. G. Wilson; S. K. Goffredi; S. B. Johnson; T. Smart; C. Widmer; C. M. Young & R. C. Vrijenhoek (2009). "Spawning and development in Osedax boneworms (Siboglinidae, Annelida)". Marine Biology. 156 (3): 395–405. Bibcode:2009MarBi.156..395R. doi:10.1007/s00227-008-1091-z. S2CID 84177994.
- ^ Goffredi, S. K.; Orphan, V. J.; Rouse, G. W.; Jahnke, L.; Embaye, T.; Turk, K.; Lee, R.; Vrijenhoek, R. C. (2005). "Evolutionary innovation: a bone-eating marine symbiosis". Environmental Microbiology. 7 (9): 1369–1378. Bibcode:2005EnvMi...7.1369G. doi:10.1111/j.1462-2920.2005.00824.x. PMID 16104860.
- ^ Piper, Ross (2007), Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals, Greenwood Press.
- ^ Rouse, G. W.; Worsaae, K.; Johnson, S.; Jones, W. J.; Vrijenhoek, R. C. (2008). "Acquisition of dwarf male 'harems' by recently settled females of Osedax roseus n. sp. (Siboglinidae; Annelida)" (PDF). Biological Bulletin. 214 (1): 67–82. doi:10.2307/25066661. JSTOR 25066661. PMID 18258777. S2CID 8457281.
- ^ Vrijenhoek, R. C.; Johnson, S.; Rouse, G. W. (2008). "Bone-eating Osedax females and their 'harems' of dwarf males are recruited from a common larval pool". Molecular Ecology. 17 (20): 4535–4544. Bibcode:2008MolEc..17.4535V. doi:10.1111/j.1365-294X.2008.03937.x. PMID 18986498. S2CID 19247165.
- ^ Vrijenhoek, R. C.; Johnson, S. B.; Rouse, G. W. (2009). "A remarkable diversity of bone-eating worms (Osedax; Siboglinidae; Annelida)". BMC Biology. 7: 74. doi:10.1186/1741-7007-7-74. PMC 2780999. PMID 19903327.
- ^ Rouse, Greg W.; Wilson, Nerida G.; Worsaae, Katrine; Vrijenhoek, Robert C. (January 2015). "A Dwarf Male Reversal in Bone-Eating Worms". Current Biology. 25 (2): 236–241. Bibcode:2015CBio...25..236R. doi:10.1016/j.cub.2014.11.032. ISSN 0960-9822. PMID 25496962.
- ^ Rouse, G. W.; Goffredi, S. K.; Vrijenhoek, R. C. (July 30, 2004). "Osedax : Bone-Eating Marine Worms with Dwarf Males". Science. 305 (5684): 668–671. Bibcode:2004Sci...305..668R. doi:10.1126/science.1098650. ISSN 0036-8075. PMID 15286372.
- ^ Miyamoto, Norio; Yamamoto, Tomoko; Yusa, Yoichi; Fujiwara, Yoshihiro (February 27, 2013). "Postembryonic development of the bone-eating worm Osedax japonicus". Naturwissenschaften. 100 (3): 285–289. Bibcode:2013NW....100..285M. doi:10.1007/s00114-013-1024-7. ISSN 0028-1042. PMID 23443811.
- ^ Miyamoto, Norio; Yamamoto, Tomoko; Yusa, Yoichi; Fujiwara, Yoshihiro (March 1, 2013). "Postembryonic development of the bone-eating worm Osedax japonicus". Naturwissenschaften. 100 (3): 285–289. Bibcode:2013NW....100..285M. doi:10.1007/s00114-013-1024-7. ISSN 1432-1904. PMID 23443811.
- ^ a b c Rouse, G. W.; Goffredi, S. K.; Vrijenhoek, R. C. (July 30, 2004). "Osedax : Bone-Eating Marine Worms with Dwarf Males". Science. 305 (5684): 668–671. Bibcode:2004Sci...305..668R. doi:10.1126/science.1098650. ISSN 0036-8075. PMID 15286372.
- ^ a b c d e f Goffredi, Shana K; Yi, Hana; Zhang, Qingpeng; Klann, Jane E; Struve, Isabelle A; Vrijenhoek, Robert C; Brown, C Titus (November 14, 2013). "Genomic versatility and functional variation between two dominant heterotrophic symbionts of deep-sea Osedax worms". The ISME Journal. 8 (4): 908–924. doi:10.1038/ismej.2013.201. ISSN 1751-7362. PMC 3960542. PMID 24225886.
- ^ a b c d e f g h i Goffredi, Shana K.; Panossian, Balig; Brzechffa, Camille; Field, Naomi; King, Chad; Moggioli, Giacomo; Rouse, Greg W.; Martín-Durán, José M.; Henry, Lee M. (June 29, 2023). Dubilier, Nicole (ed.). "A dynamic epibiont community associated with the bone-eating polychaete genus Osedax". mBio. 14 (4): e0314022. doi:10.1128/mbio.03140-22. ISSN 2150-7511. PMC 10470745. PMID 37382438.
- ^ a b c d e f g h i j k l m Moggioli, Giacomo; Panossian, Balig; Sun, Yanan; Thiel, Daniel; Martín-Zamora, Francisco M.; Tran, Martin; Clifford, Alexander M.; Goffredi, Shana K.; Rimskaya-Korsakova, Nadezhda; Jékely, Gáspár; Tresguerres, Martin; Qian, Pei-Yuan; Qiu, Jian-Wen; Rouse, Greg W.; Henry, Lee M. (May 17, 2023). "Distinct genomic routes underlie transitions to specialised symbiotic lifestyles in deep-sea annelid worms". Nature Communications. 14 (1): 2814. Bibcode:2023NatCo..14.2814M. doi:10.1038/s41467-023-38521-6. ISSN 2041-1723. PMC 10192322. PMID 37198188.
- ^ a b Goffredi, Shana K.; Johnson, Shannon B.; Vrijenhoek, Robert C. (April 2007). "Genetic Diversity and Potential Function of Microbial Symbionts Associated with Newly Discovered Species of Osedax Polychaete Worms". Applied and Environmental Microbiology. 73 (7): 2314–2323. Bibcode:2007ApEnM..73.2314G. doi:10.1128/AEM.01986-06. ISSN 0099-2240. PMC 1855680. PMID 17277220.
- ^ Miyamoto, Norio; Yoshida, Masa-aki; Koga, Hiroyuki; Fujiwara, Yoshihiro (January 13, 2017). "Genetic mechanisms of bone digestion and nutrient absorption in the bone-eating worm Osedax japonicus inferred from transcriptome and gene expression analyses". BMC Evolutionary Biology. 17 (1): 17. Bibcode:2017BMCEE..17...17M. doi:10.1186/s12862-016-0844-4. ISSN 1471-2148. PMC 5237233. PMID 28086748.
- ^ Rouse, Greg W.; Goffredi, Shana K.; Johnson, Shannon B.; Vrijenhoek, Robert C. (October 23, 2011). "Not whale-fall specialists, Osedax worms also consume fishbones". Biology Letters. 7 (5): 736–739. doi:10.1098/rsbl.2011.0202. ISSN 1744-9561. PMC 3169056. PMID 21490008.
- ^ Glover et al. 2005; Dahlgren et al. 2006; Fujijura et al. 2006
- ^ Jones et al. 2008
- ^ Rouse, GW; Goffredi, SK; Johnson, SB; Vrijenhoek, RC (October 23, 2011). "Not whale-fall specialists, Osedax worms also consume fishbones". Biology Letters. 7 (5): 736–739. doi:10.1098/rsbl.2011.0202. PMC 3169056. PMID 21490008.
- ^ Kaplan, Matt (2010). "Bone-boring worm once had a taste for birds. Osedax worms might have had a more-rounded diet 30 million years ago". Nature. doi:10.1038/news.2010.651.
- ^ Kiel, Steffen; Kahl, Wolf-Achim; Goedert, James L. (2010). "Osedax borings in fossil marine bird bones". Naturwissenschaften. 98 (1): 51–55. doi:10.1007/s00114-010-0740-5. PMC 3018246. PMID 21103978.
- ^ "Zombie worms ate plesiosaur bones". BBC News. April 15, 2015.
- ^ Alfaro-Lucas, Joan M.; Shimabukuro, Maurício; Ferreira, Giulia D.; Kitazato, Hiroshi; Fujiwara, Yoshihiro; Sumida, Paulo Y.G. (December 2017). "Bone-eating Osedax worms (Annelida: Siboglinidae) regulate biodiversity of deep-sea whale-fall communities". Deep Sea Research Part II: Topical Studies in Oceanography. 146: 4–12. Bibcode:2017DSRII.146....4A. doi:10.1016/j.dsr2.2017.04.011.
- ^ a b Danise, Silvia; Higgs, Nicholas D. (April 2015). "Bone-eating Osedax worms lived on Mesozoic marine reptile deadfalls". Biology Letters. 11 (4): 20150072. doi:10.1098/rsbl.2015.0072. ISSN 1744-9561. PMC 4424620. PMID 25878047.
- ^ Kiel, Steffen; Kahl, Wolf-Achim; Goedert, James L. (March 2013). "Traces of the bone-eating annelid Osedax in Oligocene whale teeth and fish bones". Paläontologische Zeitschrift. 87 (1): 161–167. Bibcode:2013PalZ...87..161K. doi:10.1007/s12542-012-0158-9. ISSN 0031-0220.
- ^ WoRMS, Genus Osedax
- ^ Rouse, Greg W.; Goffredi, Shana K.; Johnson, Shannon B.; Vrijenhoek, Robert C. (February 5, 2018). "An inordinate fondness for Osedax (Siboglinidae: Annelida): Fourteen new species of bone worms from California". Zootaxa. 4377 (4): 451–489. doi:10.11646/zootaxa.4377.4.1. PMID 29690036. S2CID 13854107.
- ^ Fujiwara, Yoshihiro; Jimi, Naoto; Sumida, Paulo Y. G.; Kawato, Masaru; Kitazato, Hiroshi (August 1, 2019). "New species of bone-eating worm Osedax from the abyssal South Atlantic Ocean (Annelida, Siboglinidae)". ZooKeys (814): 53–69. Bibcode:2019ZooK..814...53F. doi:10.3897/zookeys.814.28869. PMC 6333729. PMID 30651712.
- ^ Fujikura, Fujiwara & Kawato. ZOOLOGICAL SCIENCE 23: 733–740 (2006)
Further reading edit
- Jones, W. J; Johnson, S. B; Rouse, G. W; Vrijenhoek, R. C (February 22, 2008). "Marine worms (genus Osedax) colonize cow bones". Proceedings of the Royal Society B: Biological Sciences. 275 (1633): 387–391. doi:10.1098/rspb.2007.1437. PMC 2596828. PMID 18077256.
- A. G. Glover; K. M. Kemp; C. R. Smith; T. G. Dahlgren (2008). "On the role of bone-eating worms in the degradation of marine vertebrate remains". Proceedings of the Royal Society B. 275 (1646): 1959–1961. doi:10.1098/rspb.2008.0177. PMC 2596369. PMID 18505721.
- R. C. Vrijenhoek, P. Collins and C. L. Van Dover (2008). "Bone-eating marine worms: habitat specialists of generalists?". Proceedings of the Royal Society B. 275 (1646): 1963–1964. doi:10.1098/rspb.2008.0350. PMC 2596361.
External links edit
- Press release describing discovery of Osedax
- BBC website – link to story about discovery of Osedax worms in the North Sea
- A Motley Collection of Boneworms – Monterey Bay Aquarium Research Institute
- Discovered in the deep: the worm that eats bones – The Guardian