Helenin is a phytochemical mixture found in many plant species, including the Inula helenium (elecampane) of the family Asteraceae. It is a mixture of two isomeric sesquiterpene lactones, alantolactone and isoalantolactone.
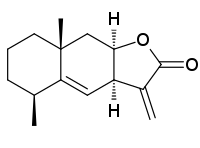 Alantolactone
| |
 Isoalantolactone
| |
| Names | |
|---|---|
| IUPAC names
Alantolactone: (3aR,5S,8aR,9aR)-5,8a-Dimethyl-3-methylene-3a,5,6,7,8,8a,9,9a-octahydronaphtho[2,3-b]furan-2(3H)-one
Isoalantolactone: (3aR,4aS,8aR,9aR)-8a-Methyl-3,5-bis(methylene)decahydronaphtho[2,3-b]furan-2(3H)-one | |
| Other names
Elecampane camphor, Inula camphor, Alant camphor
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C15H20O2 | |
| Molar mass | 232.323 g·mol−1 |
| Appearance | Crystalline powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In 1895 the German scientists Julius Bredt and Wilhelm Posh extracted helenin from Inula helenium and determined its physical and chemical properties.[1]
Natural sources edit
Alantolactone occurs in the roots of Inula helenium and other Inula species.[2]
Properties edit
Helenin can be extracted from the roots of Inula helenium using alcohol or other non-polar solvents to produce a mixture with a composition of about 40% alantolactone and 60% isoalantolactone.[3]
Biological activity edit
Alantolactone has a variety of in vitro biochemical properties, including:
Toxicity edit
Certain individuals have experienced contact dermatitis when exposed to alantolactone.[9]
References edit
- ^ Chemical Society (Great Britain) (1895). The collected works of Sir Humphry Davy ...: Discourses delivered before the Royal society. Elements of agricultural chemistry, pt. I. Smith, elder and Company. p. 555. Retrieved 31 July 2015.
- ^ Hoffmann, David (2003). Medical Herbalism:The Science and Practice of Herbal Medicine. Health & Fitness. ISBN 978-1594778902.
- ^ Xu, Renjie (2014). "Pharmacokinetic Comparison of Isoalantolactone and Alantolactone in Rats after Administration Separately by Optimization of an UPLC-MS2 Method". Journal of Chemistry. 2014: 1–8. doi:10.1155/2014/354618.
- ^ Zhao, Peng (19 Jan 2015). "Alantolactone Induces Apoptosis and Cell Cycle Arrest on Lung Squamous Cancer SK-MES-1 Cells". Journal of Biochemical and Molecular Toxicology. 29 (5): 199–226. doi:10.1002/jbt.21685. PMID 25597476. S2CID 10440798.
- ^ Chun, J (1 Feb 2015). "Alantolactone selectively suppresses STAT3 activation and exhibits potent anticancer activity in MDA-MB-231 cells". Cancer Letters. 357 (1): 393–403. doi:10.1016/j.canlet.2014.11.049. PMID 25434800.
- ^ Hye, Sun Lim (17 Apr 2015). "Alantolactone from Saussurea lappa Exerts Antiinflammatory Effects by Inhibiting Chemokine Production and STAT1 Phosphorylation in TNF-α and IFN-γ-induced in HaCaT cells". Phytotherapy Research. 29 (7): 1088–1096. doi:10.1002/ptr.5354. PMID 25881570. S2CID 5196219.
- ^ Alejandro, Barreroa (2000). "New sources and antifungal activity of sesquiterpene lactones". Fitoterapia. 66 (71): 60–64. doi:10.1016/s0367-326x(99)00122-7. PMID 20095126.
- ^ O'Shea, S (2009). "In vitro activity of Inula helenium against clinical Staphylococcus aureus strains including MRSA". British Journal of Medical Science. 66 (4): 186–9. doi:10.1080/09674845.2009.11730271. PMID 20095126.
- ^ Stampf, J (August 1978). "Allergic contact dermatitis due to sesquiterpene lactones. A comparative study of human and animal sensitivity to alpha-methylene-gamma-butyrolactone and derivatives". The British Journal of Dermatology. 99 (2): 163–9. doi:10.1111/j.1365-2133.1978.tb01977.x. PMID 698105. S2CID 73218116.