Disodium tetracarbonylferrate is the organoiron compound with the formula Na2[Fe(CO)4]. It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation.[1] An oxygen-sensitive colourless solid, it is a reagent in organometallic and organic chemical research. The dioxane solvated sodium salt is known as Collman's reagent, in recognition of James P. Collman, an early popularizer of its use.[2]
| |
| Names | |
|---|---|
| IUPAC name
disodium tetracarbonylferrate
| |
| Systematic IUPAC name
disodium tetracarbonylferrate | |
| Other names
disodium iron tetracarbonyl,
Collman's reagent
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.035.395 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4FeNa2O4 | |
| Molar mass | 213.87 |
| Appearance | Colorless solid |
| Density | 2.16 g/cm3, solid |
| Decomposes | |
| Solubility | tetrahydrofuran, dimethylformamide, dioxane |
| Structure | |
| Distorted tetrahedron | |
| Tetrahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Pyrophoric |
| Related compounds | |
Related compounds
|
Iron pentacarbonyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
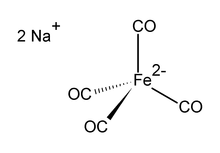
Structure
editThe dianion [Fe(CO)4]2− is isoelectronic with Ni(CO)4.[3][4] The iron center is tetrahedral, with Na+---OCFe interactions. It is commonly used with dioxane complexed to the sodium cation.
Synthesis
editThe reagent was originally generated in situ by reducing iron pentacarbonyl with sodium amalgam.[5] Modern synthesis use sodium naphthalene or sodium benzophenone ketyls as the reducants:[1][6]
- Fe(CO)5 + 2 Na → Na2[Fe(CO)4] + CO
When a deficiency of sodium is used, the reduction affords deep yellow octacarbonyl diferrate:[1]
- 2 Fe(CO)5 + 2 Na → Na2[Fe2(CO)8] + 2 CO
Some specialized methods do not start with iron carbonyl.[7]
Reactions
editIt is used to synthesise aldehydes from alkyl halides.[8] The reagent was originally described for the conversion of primary alkyl bromides to the corresponding aldehydes in a two-step, "one-pot" reaction:[5]
- Na2[Fe(CO)4] + RBr → Na[RFe(CO)4] + NaBr
This solution is then treated sequentially with PPh3 and then acetic acid to give the aldehyde, RCHO.
Disodium tetracarbonylferrate can be used to convert acyl chlorides to aldehydes. This reaction proceeds via the intermediacy of iron acyl complex.
- Na2[Fe(CO)4] + RCOCl → Na[RC(O)Fe(CO)4] + NaCl
- Na[RC(O)Fe(CO)4] + HCl → RCHO + "Fe(CO)4" + NaCl
Disodium tetracarbonylferrate reacts with alkyl halides (RX) to produce alkyl complexes:
- Na2[Fe(CO)4] + RX → Na[RFe(CO)4] + NaX
Such iron alkyls can be converted to the corresponding carboxylic acid and acid halides:
- Na[RFe(CO)4] + O2, H+ →→ RCO2H + Fe...
- Na[RFe(CO)4] + 2 X2 → RC(O)X + FeX2 + 3 CO + NaX
References
edit- ^ a b c Strong, H.; Krusic, P. J.; San Filippo, J. (1990). Sodium Carbonyl Ferrates, Na2[Fe(CO)4], Na2[Fe2(CO)8], and Na2[Fe3(CO)11]. Bis[μ-Nitrido-Bis(triphenylphosphorus)1+] Undeca-Carbonyltriferrate2−, [(Ph3P)2N]2[Fe3(CO)11]. Inorganic Syntheses. Vol. 28. pp. 203–207. doi:10.1002/9780470132593.ch52. ISBN 0-471-52619-3.
- ^ Miessler, G. L.; Tarr, D. A. (2004). Inorganic Chemistry. Upper Saddle River, NJ: Pearson.
- ^ Chin, H. B.; Bau, R. (1976). "The Crystal Structure of Disodium Tetracarbonylferrate. Distortion of the Tetracarbonylferrate2− Anion in the Solid State". Journal of the American Chemical Society. 98 (9): 2434–2439. doi:10.1021/ja00425a009.
- ^ Teller, R. G.; Finke, R. G.; Collman, J. P.; Chin, H. B.; Bau, R. (1977). "Dependence of the tetracarbonylferrate(2-) geometry on counterion: crystal structures of dipotassium tetracarbonylferrate and bis(sodium crypt) tetracarbonylferrate [crypt = N(CH2CH2OCH2CH2OCH2CH2)3N]". Journal of the American Chemical Society. 99 (4): 1104–1111. doi:10.1021/ja00446a022.
- ^ a b Cooke, M. P. (1970). "Facile Conversion of Alkyl Bromides into Aldehydes Using Sodium Tetracarbonylferrate(-II)". Journal of the American Chemical Society. 92 (20): 6080–6082. doi:10.1021/ja00723a056.
- ^ Richard G. Finke, Thomas N. Sorrell (1979). "Nucleophilic Acylation with Disodium Tetracarbonylferrate: Methyl 7-Oxoheptanoate and Methyl 7-Oxoöctanoate". Organic Syntheses. 59: 102. doi:10.15227/orgsyn.059.0102.
- ^ Scholsser, M. (2013). Organometallics in Synthesis, Third Manual. Chicester, England: Wiley.
- ^ Pike, R. D. (2001). "Disodium Tetracarbonylferrate(-II)". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd465.
Further reading
edit- Collman, J. P. (1975). "Disodium Tetracarbonylferrate, a Transition Metal Analog of a Grignard Reagent". Accounts of Chemical Research. 8 (10): 342–347. doi:10.1021/ar50094a004.
- Ungurenasu, C.; Cotzur, C. (1982). "Disodium Tetracarbonylferrate: A Reagent for Acid Functionalization of Halogenated Polymers". Polymer Bulletin. 6 (5–6): 299–303. doi:10.1007/BF00255401. S2CID 101154955.
- Hieber, V. W.; Braun, G. (1959). "Notizen: "Rheniumcarbonylwasserstoff" und Methylpentacarbonylrhenium". Zeitschrift für Naturforschung B. 14 (2): 132–133. doi:10.1515/znb-1959-0214. S2CID 94402946.