Aumolertinib (trade name Ameile) is a pharmaceutical drug for the treatment of cancer.[1] It is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI).[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Ameile |
| Other names | Almonertinib; HS-10296 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| Chemical and physical data | |
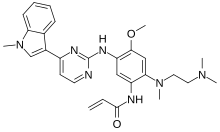
| Formula | C30H35N7O2 |
| Molar mass | 525.657 g·mol−1 |
In China, aumolertinib is approved for the treatment of patients with EGFR T790M mutation—positive non-small-cell lung cancer (NSCLC) who have progressed on or after other EGFR TKI therapy.[3][4]
References edit
- ^ Shirley M, Keam SJ (April 2022). "Aumolertinib: A Review in Non-Small Cell Lung Cancer". Drugs. 82 (5): 577–584. doi:10.1007/s40265-022-01695-2. PMID 35305259.
- ^ Johnson ML, Miller VA, Patel S, Zhao Y, Cheng L, Ali SM, et al. "Aumolertinib with chemotherapy or alone compared with osimertinib in patients with EGFR-mutant non–small-cell lung cancer (TREBLE)". Journal of Clinical Oncology. 41 (16). doi:10.1200/JCO.2023.41.16_suppl.TPS915 (inactive 2024-04-17).
{{cite journal}}: CS1 maint: DOI inactive as of April 2024 (link) - ^ "Almonertinib Approved in China for EGFR T79M+ NSCLC". onclive.com. March 19, 2020.
- ^ Lu S, Wang Q, Zhang G, Dong X, Yang CT, Song Y, et al. (March 2022). "Efficacy of Aumolertinib (HS-10296) in Patients With Advanced EGFR T790M+ NSCLC: Updated Post-National Medical Products Administration Approval Results From the APOLLO Registrational Trial". Journal of Thoracic Oncology. 17 (3): 411–422. doi:10.1016/j.jtho.2021.10.024. PMID 34801749.