This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (May 2024) |
1,2-Dibromotetrachloroethane (DBTCE) is an organohalide with the chemical formula C2Br2Cl4. It is a crystalline solid that emits lachrymatory (tear-producing) vapours.[2] Dibromotetrachloroethane can be used as a fungicide,[2] flame retardant[3] and a source for bromine in the laboratory.[4] Because the 1,1-dibromotetrachloroethane isomer is rare, 1,2-dibromotetrachloroethane is frequently referred to as simply dibromotetrachloroethane.
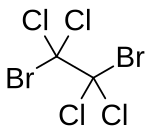
| |
| Names | |
|---|---|
| IUPAC name
1,2-dibromo-1,1,2,2-tetrachloroethane
| |
| Other names
dibromotetrachloroethane, sym-Dibromotetrachloroethane, DBTCE, Bromure de chloréthose ("bromide of chlorethose")
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.010.125 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2Br2Cl4 | |
| Molar mass | 325.63 g·mol−1 |
| Appearance | Crystalline solid |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Reactions and synthesis
editDibromotetrachloroethane decomposes to Tetrachloroethylene and bromine when heated. Reacted with potassium sulphide, it gives tetrachloroethylene, potassium bromide and sulphur:[5]
- C2Br2Cl4 + K2S → C2Cl4 + S + 2 KBr
Dibromotetrachloroethane, when reacted with aniline at 140 to 150 °C, gives pure tetrachloroethylene.[6]
When reacted with compounds like cyclohexene, 2,2,4-trimethylpentl-ene, 1-hexene, 1-octene, 2-methyl-1-butene and 2,2,4-trimethyl-2-pentene, it yields allylic monobromides via bromination. Dibromotetrachloroethane loses both of its bromine atoms, leaving tetrachloroethylene and hydrogen bromide.[7]
Dibromotetrachloroethane was discovered by the Italian chemist Faustino Malaguti in 1846.[8] Malaguti exposed a mixture of Tetrachloroethylene (then known as chloréthose) and bromine to sunlight. It was named Bromure de chloréthose ("bromide of chlorethose") after its synthesis method.[5] Similar to Malaguti's method, modern synthesis of dibromotetrachloroethane uses bromine dissolved in carbon tetrachloride.
References
edit- ^ "1,2-Dibromotetrachloroethane". pubchem.ncbi.nlm.nih.gov.
- ^ a b Dewayne Torgeson, Agricultural and Industrial Applications Environmental Interactions: An Advanced Treatise (2012), p. 333
- ^ Richard Montgomery Stephenson, Stanisław Malanowski, Handbook of the Thermodynamics of Organic Compounds (2012), p.20
- ^ B. Iddon, Basil John Wakefield, D. Price, Bromine Compounds: Chemistry and Applications (1988)
- ^ a b Edmond Frémy, Paul Louis Chastain, Encyclopédie Chimique (1883) p. 235
- ^ E. Burgoin, Preparation of Perchlorethylene (1870)
- ^ G. H. Williams Advances in Free-radical Chemistry (1972), p. 186
- ^ Faustino Malaguti, Recherches sur Éthers Chlorés, Annales de Chimie et de Physique, 16-3, p. 24