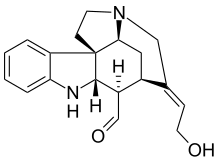
The so-called Wieland-Gumlich aldehyde (6) is an indoline derived by chemical degradation from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 4 steps from strychnine (1)[2][3][4] by Walter Gumlich and Koozoo Kaziro working in the laboratory of Heinrich Wieland. This degradation study was part of an attempt to elucidate the chemical structure of strychnine.
| |
| Names | |
|---|---|
| IUPAC name
(1S,9S,10R,11R,12E,17S)-12-(2-hydroxyethylidene)-8,14-diazapentacyclo[9.5.2.01,9.02,7.014,17]octadeca-2,4,6-triene-10-carbaldehyde
| |
| Other names
Caracurine VII, Deacetyldiaboline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C19H22N2O2 | |
| Molar mass | 310.397 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
This degradation takes place through conversion of strychnine to the oxime 2 using amyl nitrite, Beckmann fragmentation of 2 to the carbamic acid 3 by use of thionyl chloride, decarboxylation of 3 to nitrile 4, and nucleophilic displacement of cyanide by barium hydroxide to give hemiacetal 5, which is in equilibrium with the Wieland-Gumlich aldehyde (6).
The Wieland-Gumlich aldehyde reverts to strychnine in a single reaction using malonic acid, acetic anhydride and sodium acetate in acetic acid.[5]
The Wieland-Gumlich aldehyde has been used in the industrial synthesis of alcuronium chloride (Alloferin) via dimerization.[6]
References
edit- ^ Chambers, Michael. "ChemIDplus - 466-85-3 - UFUDXCDPABDFHK-SRCYXDNASA-N - Wieland-gumlich aldehyde - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.nlm.nih.gov.
- ^ Wieland, H.; Gumlich, W. (1932) Über einige neue Reaktionen der Strychnos - Alkaloide. XI Justus Liebigs Annalen der Chemie 494(1):191-200. (original report of fragmentation of the strychnine lactam ring)
- ^ Wieland H.; Kaziro, K. (1933) Abbauversuche vom Isonitroso-strychnin aus. Über Strychnos-Alkaloide. XIII Justus Liebigs Annalen der Chemie 506(1):60–76. (definitive characterization of the Wieland-Gumlich aldehyde)
- ^ Witkop B. (1992) Remembering Heinrich Wieland (1877-1957) Portrait of an Organic Chemist and Founder of Modern Biochemistry Med. Res. Rev. 12(3):195-274. (Witkop notes [p. 220] that "Wieland and Kaziro studied the Beckmann rearrangement of this oxime and the loss of hydrogen cyanide to yield an aldehyde, that should correctly be called Wieland-Kaziro aldehyde, but became known and accepted as Wieland-Gumlich aldehyde...")
- ^ F. A. L. Anet, R. Robinson, Chem. Ind. (London) 1953, 245.
- ^ Alkaloids: Nature's Curse or Blessing? Manfred Hesse. Wiley, 2002. See pp. 230-232. ISBN 978-3-90639-024-6
