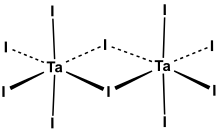
Tantalum(V) iodide is the inorganic compound with the formula Ta2I10. Its name comes from the compound's empirical formula, TaI5.[2] It is a diamagnetic, black solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two TaI5 units are joined by a pair of iodide bridges. There is no bond between the Ta centres.[3] Niobium(V) chloride, niobium(V) bromide, niobium(V) iodide, tantalum(V) chloride, and tantalum(V) bromide all share this structural motif.
| |
| Names | |
|---|---|
| Other names
Tantalum pentaiodide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Ta2I10 | |
| Molar mass | 1631 |
| Appearance | black solid |
| Density | 5.8 g/cm3 |
| Melting point | 382[1] °C (720 °F; 655 K) sublimes |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H331 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P321, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis and structure
editTantalum pentaiodide forms from the reaction of tantalum pentoxide with aluminium triiodide:[4]
- 3 Ta2O5 + 10 AlI3 → 6 TaI5 + 5 Al2O3
References
edit- ^ McCarley, R. E.; Boatman, J.C. (1965). "The Equilibrium Phase Diagrams for the Tantalum-Tantalum Bromide and Tantalum-Tantalum Iodide Systems". Inorganic Chemistry. 4 (10): 1486–1491. doi:10.1021/ic50032a029. S2CID 67778953.
- ^ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Müller, U. (1979). "Die Kristallstruktur von Tantalpentajodid und ihre Fehlordnung". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 35 (11): 2502–2509. Bibcode:1979AcCrB..35.2502M. doi:10.1107/S0567740879009778.
- ^ G. Braurer (1963). "Niobium(V) and Tantalum(V) Bromides". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY, NY: Academic Press. p. 1311.