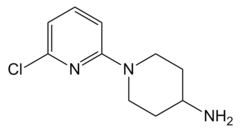
SR-57227 is a potent and selective agonist at the 5HT3 receptor, with high selectivity over other serotonin receptor subtypes and good blood–brain barrier penetration.[1][2][3][4]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.163.915 |
| Chemical and physical data | |
| Formula | C10H14ClN3 |
| Molar mass | 211.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
edit- ^ Bachy A, Héaulme M, Giudice A, Michaud JC, Lefevre IA, Souilhac J, et al. (June 1993). "SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo". European Journal of Pharmacology. 237 (2–3): 299–309. doi:10.1016/0014-2999(93)90282-M. PMID 7689975.
- ^ Maksay G, Simonyi M, Bikádi Z (October 2004). "Subunit rotation models activation of serotonin 5-HT3AB receptors by agonists". Journal of Computer-Aided Molecular Design. 18 (10): 651–64. doi:10.1007/s10822-004-6259-0. PMID 15849995. S2CID 10510254.
- ^ Yoo JH, Cho JH, Yu HS, Lee KW, Lee BH, Jeong SM, et al. (November 2006). "Involvement of 5-HT receptors in the development and expression of methamphetamine-induced behavioral sensitization: 5-HT receptor channel and binding study". Journal of Neurochemistry. 99 (3): 976–88. doi:10.1111/j.1471-4159.2006.04137.x. PMID 16942594. S2CID 38685481.
- ^ Li Y, Raaby KF, Sánchez C, Gulinello M (November 2013). "Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats". Behavioural Brain Research. 256: 520–8. doi:10.1016/j.bbr.2013.09.002. PMID 24016840. S2CID 45617581.