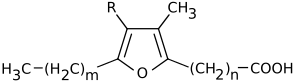
Furan fatty acids are a group of fatty acids that contain a furan ring. To this furan ring, an unbranched carboxylic acid and, at another position, an alkyl residue are attached. Natural furan fatty acids are mono- or di-methylated on the furan ring.[1] Furan fatty acids can be found in a variety of plant and animal species.
Carboxy-substituted furan fatty acids are known as urofuran acids. Urofuran fatty acids are metabolic products of furan fatty acids and can be detected, for example, in human urine.[2]
Occurrence
editFuran fatty acids are found mainly in the liver fat of fish, in crustaceans and horn corals. They can also be found in the liver of cattle and rats, as well as in human blood; either in free form or in triglycerides or esterified to cholesterol. In fish, the concentration of furan fatty acids is particularly high in the liver after hunger periods.
Furan fatty acids can be detected in a variety of organisms and products such as butter and butter oil.[3] It is now assumed that this class of compounds is ubiquitous.[4][5]
Furan fatty acids in animals are based on the uptake and accumulation of furan fatty acids from plant constituents.[6] In human blood, the total furan fatty acid content is about 50 ng/ml. Per day, a person separates between 0.5 and 3 mg of urofuran acids - the metabolic product of the furans acids.[7][8][9] Animals are not able to synthesize furan fatty acids. Larger amounts of furan fatty acids are produced mainly by algae, but also some plants and microorganisms. These serve fish and mammals as food. The furan fatty acids thus absorbed are incorporated into phospholipids and cholesterol esters.[8]
Function and physiological effects
editFuran fatty acids are reactive compounds. They are easily oxidizable by photooxidation,[10] autoxidation,[10][11][12] or catalyzed by lipoxygenase-1.[10][13][14] Upon the exposure to light, the aroma 3-methyl-2,4-nonanedione (MND) is formed from furan fatty acids in the reaction with singlet oxygen, which has a hay-like odor and is found, for example, in green tea.[15][16]
Furan fatty acids are very effectively acting as radical scavengers. In this process dioxoenoic fatty acids are formed, which are by themselves very unstable and form thioethers with thiols such as cysteine or glutathione.[17] As potent antioxidants, they specifically trap hydroxyl radicals.[18] It is therefore believed that this is in different biological systems their main function.[19] They also inhibit the singlet oxygen-induced hemolysis of red blood cells (red blood cell disintegration).[20][21]
Plants and algae produce furan fatty acids during the biosynthesis from polyunsaturated fatty acids (PUFA). These are seemingly used in these organisms as protection against free radicals generated by sunlight.[22][23]
Occasionally it is speculated that the health-promoting properties originally attributed to omega-3 fatty acids may not be based on themself, but on the furan fatty acids also present in the fish.[24][25] A clinical trial of isolated omega-3 fatty acids such as eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) in patients who have had a myocardial infarction previously showed no significant difference in cardiovascular effects compared to a placebo.[26]
The exact pathological effects of furan fatty acids have not yet been clarified in detail and are the subject of current research. In addition to the antioxidant effect, anti-tumor (against malignant tumors) and antithrombotic effects (anti-thrombosis) are also suspected.[27] In 2002, xenohormonal properties were observed for the two furan fatty acids 9,(12)-oxy-10,13-dihydroxystearic acid and 10,(13)-oxy-9,12-dihydroxystearic acid. In vitro experiments on MCF-7 cells (breast cancer cells with estrogen receptor) revealed mitogenic properties as well as an influence on estrus. In the latter case, the transition to metestrus was initiated.[28][29] In vivo, a reduction in mating willingness was observed after intake of furan fatty acids on female color rats.[30] However, neither estrogen nor anti-estrogenic activity has been demonstrated.[28][29][31] Chickens were found to have no adverse effects on feeding, fertility, egg weight, eggshell thickness, and other reproductive parameters after the intake of furan fatty acids.[31]
History
editFuran fatty acids were first detected in 1966 by L. J. Morris and colleagues as part of an oil derived from seeds of Exocarpus cupressiformis (a sandalwood-type plant).[32] Years later, other analysis methods showed that the furan fatty acid 9,12-epoxyoctadeca-9,11-dienoic acid was in fact not contained in the oil of Exocarpus cupressiformis as described by Morris. Instead, it was formed during the sample preparation used by Morries and colleagues for the argentation chromatography by oxidation of hydroxyfatty acids, in a base-catalyzed transesterification.[33] In 1974, furan fatty acids were first identified in pike (Esox lucius) by Robert L. Glass and colleagues using coupled gas chromatography–mass spectrometry (GC-MS).[5][34]
Literature
edit- N. Hinrichsen: "Synthese und Analytik von Furanfettsäuren". Dissertation, Universität Hamburg, 2009, ISBN 3-86853-028-2
- Dembitsky, V. M.; Rezanka, T. (1996). "Furan fatty acids of some brackish invertebrates from the Caspian sea". Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 114 (3): 317–320. doi:10.1016/0305-0491(96)00063-6.
- Prinsep, MR; Blunt, JW; Munro, MH (1994). "Isolation of the furan fatty acid, (8Z,11Z,14Z,17Z)-3,6-epoxyeicos-3,5,8,11,14,17-hexenoic acid from the New Zealand sponge Hymeniacidon hauraki". J Nat Prod. 57 (11): 1557–9. doi:10.1021/np50113a014. PMID 7853004.
- Wahl, H. G. (1988). "Die Bedeutung von Furanfettsäuren als Inhaltsstoffe von Fischölpräparaten". GIT Labor-Fachzeitschrift. 4: 368–372.
- Ishii, K; Okajima, H; Okada, Y; Watanabe, H (1988). "Studies on furan fatty acids of salmon roe phospholipids". J Biochem. 103 (5): 836–9. doi:10.1093/oxfordjournals.jbchem.a122356. PMID 3182753.
- Spiteller, G. (1987). "Furanfettsäuren". Nachrichten aus Chemie, Technik und Laboratorium. 35 (12): 1240–1243. doi:10.1002/nadc.19870351204.
- Rahn, C. H.; et al. (1979). "Synthesis of naturally occurring furan fatty acids". The Journal of Organic Chemistry. 44 (19): 3420–3424. doi:10.1021/jo01333a036.
- Glass, RL; Krick, TP; Sand, DM; Rahn, CH; Schlenk, H (1975). "Furanoid fatty acids from fish lipids". Lipids. 10 (11): 695–702. doi:10.1007/bf02532763. PMID 1196019. S2CID 4052488.
- R. Jónsdóttir, P. Hamaguchi, G. Ólafsdóttir, T. Wang: "Antioxidants from Icelandic marine sources." (PDF-File; 429 kB), May 2010.
References
edit- ^ S. Göckler: "Metabolismus und genetische Toxizität von Furanfettsäuren, sowie deren Einfluss auf Zellmembranen in vitro". Dissertation, Universität Karlsruhe, 2009.
- ^ Sand, DM; Schlenk, H; Thoma, H; Spiteller, G (1983). "Catabolism of fish furan fatty acids to urofuran acids in the rat". Biochim Biophys Acta. 751 (3): 455–61. doi:10.1016/0005-2760(83)90306-5. PMID 6849955.
- ^ Guth, H.; Grosch, W. (1992). "Furan fatty acids in butter and butter oil". Zeitschrift für Lebensmittel-Untersuchung und Forschung. 194 (4): 360–362. doi:10.1007/BF01193220. S2CID 83109872.
- ^ Hannemann, K; Puchta, V; Simon, E; Ziegler, H; Ziegler, G; Spiteller, G (1989). "The common occurrence of furan fatty acids in plants". Lipids. 24 (4): 296–298. doi:10.1007/BF02535166. PMID 2755307. S2CID 4061080.
- ^ a b R. Pompizzi: "Furanfettsäuren als Vorläufer von Aromastoffen". Dissertation, ETH Zürich, 1999.
- ^ Gorst-Allman, C. P.; et al. (1988). ""Investigations of the origin of the furan fatty acids (F-acids)". In". Lipids. 23 (11): 1032–1036. doi:10.1007/BF02535648. PMID 3237002. S2CID 4031863.
- ^ Gerhard Spiteller (1987), "Furanfettsäuren", Nachrichten aus Chemie, Technik und Laboratorium (in German), vol. 35, no. 12, pp. 1240–1243, doi:10.1002/nadc.19870351204
- ^ a b c Spiteller, G. (2005). ""Furan fatty acids: Occurrence, synthesis, and reactions. Are furan fatty acids responsible for the cardioprotective effects of a fish diet?" In". Lipids. 40 (8): 755–771. doi:10.1007/s11745-005-1438-5. PMID 16296395. S2CID 4043362.
- ^ Simon Göckler (2009), Metabolismus und genetische Toxizität von Furanfettsäuren, sowie deren Einfluss auf Zellmembranen in vitro (Dissertation) (in German), Karlsruhe: Universität Karlsruhe, p. 11, nbn:de:swb:90-1060301013696247
- ^ a b c Boyer, RF; Litts, D; Kostishak, J; Wijesundera, RC; Gunstone, FD (1979). "The action of lipoxygenase-1 on furan derivatives". Chem Phys Lipids. 25 (3): 237–46. doi:10.1016/0009-3084(79)90109-9. PMID 119581.
- ^ Ishii, K.; et al. (1988). ""The Composition of Furan Fatty Acids in the Crayfish". In". Lipids. 23 (7): 694–700. doi:10.1007/BF02535671. PMID 27520122. S2CID 4045198.
- ^ Rosenblat, G.; et al. (1993). "Inhibition of Bacterial Urease by Autoxidation of Furan C-18 Fatty Acid Methyl Ester Products". Journal of the American Oil Chemists' Society. 70 (5): 501–505. doi:10.1007/BF02542584. S2CID 85295211.
- ^ A. Batna und G. Spiteller: "Oxidation of Furan Fatty Acids by Soybean Lipoxygenase-1 in the Presence of Linoleic Acid". In: Chemistry and Physics of Lipids 1994; 70, S. 179–185, 8033289.
- ^ Batna, A; Spiteller, G (1994). "Effects of soybean lipoxygenase-1 on phosphatidylcholines containing furan fatty acids". Lipids. 29 (6): 397–403. doi:10.1007/bf02537308. PMID 8090060. S2CID 4014848.
- ^ Werner Grosch (2010-11-26). "Das Geheimnis des Tee-Aromas" (PDF). Teeverband.de (in German). Deutsches Tee-Institut. Archived from the original (PDF; 98 kB) on 2016-04-18. Retrieved 2017-02-20.
- ^ W. Grosch u. a.: Lehrbuch der Lebensmittelchemie Verlag Springer, 2007, ISBN 3-540-73201-2, S. 987. [1], p. 987, at Google Books
- ^ Jandke, Joachim; Schmidt, Jochen; Spiteller, Gerhard (1988). ""Ueber das Verhalten von F-Saeuren bei Oxidation mit Lipoxydase in Anwesenheit von SH-haltigen Verbindungen". In". Liebigs Annalen der Chemie. 1988: 29–34. doi:10.1002/jlac.198819880107.
- ^ Spiteller, G (2008). "Peroxyl radicals are essential reagents in the oxidation steps of the Maillard reaction leading to generation of advanced glycation end products". Ann N Y Acad Sci. 1126 (1): 128–33. Bibcode:2008NYASA1126..128S. doi:10.1196/annals.1433.031. PMID 18448806. S2CID 10696198.
- ^ Okada, Y; Kaneko, M; Okajima, H (1996). "Hydroxyl radical scavenging activity of naturally occurring furan fatty acids". Biol Pharm Bull. 19 (12): 1607–10. doi:10.1248/bpb.19.1607. PMID 8996648.
- ^ Okada, Y; Okamjima, H; Terauchi, M; Konishi, H; Liu, IM; Watanabe, H (1990). "[Inhibitory effects of naturally occurring furan fatty acids on hemolysis of erythrocytes induced by singlet oxygen]". Yakugaku Zasshi. 110 (9): 665–72. doi:10.1248/yakushi1947.110.9_665. PMID 2175788.
- ^ White, D. C.; et al. (2005). "Phospholipid furan fatty acids and ubiquinone-8: lipid biomarkers that may protect dehalococcoides strains from free radicals". Applied and Environmental Microbiology. 71 (12): 8426–8433. Bibcode:2005ApEnM..71.8426W. doi:10.1128/AEM.71.12.8426-8433.2005. PMC 1317454. PMID 16332831.
- ^ Spiteller, G (2007). "The important role of lipid peroxidation processes in aging and age dependent diseases". Mol Biotechnol. 37 (1): 5–12. doi:10.1007/s12033-007-0057-6. PMID 17914157. S2CID 28103222.
- ^ G. G. Habermehl u. a. Naturstoffchemie Verlag Springer, 2008, ISBN 3-540-73732-4, S. 566 [2], p. 566, at Google Books
- ^ E. Bodderas: "Das Märchen vom guten Fett". In: Die Welt, 30 May 2010
- ^ Bodderas, Elke (2010-05-31). "Omega-3-Fette nicht gesünder als Schweineschmalz". Welt Online. Retrieved 2012-08-05.
- ^ Kromhout, D; Giltay, EJ; Geleijnse, JM (2010). "n-3 fatty acids and cardiovascular events after myocardial infarction". N Engl J Med. 363 (21): 2015–26. doi:10.1056/NEJMoa1003603. PMID 20929341.
- ^ Deborah Pacetti, Francesca Alberti, Emanuele Boselli, Natale G. Frega (2010), "Characterisation of furan fatty acids in Adriatic fish", Food Chemistry, vol. 122, no. 1, pp. 209–215, doi:10.1016/j.foodchem.2010.02.059
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Markaverich, BM; Alejandro, MA; Markaverich, D; Zitzow, L; Casajuna, N; Camarao, N; Hill, J; Bhirdo, K; Faith, R; Turk, J; Crowley, JR (2002). "Identification of an endocrine disrupting agent from corn with mitogenic activity". Biochem Biophys Res Commun. 291 (3): 692–700. doi:10.1006/bbrc.2002.6499. PMID 11855846.
- ^ a b Markaverich, B; Mani, S; Alejandro, MA; Mitchell, A; Markaverich, D; Brown, T; Velez-Trippe, C; Murchison, C; O'Malley, B; Faith, R (2002). "A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells". Environ Health Perspect. 110 (2): 169–77. doi:10.1289/ehp.02110169. PMC 1240732. PMID 11836146.
- ^ Schettler, T. (2003). ""Corn and corn-derived products: Sources of endocrine disruptors". In". Environmental Health Perspectives. 111 (13): A691. doi:10.1289/ehp.111-a691. PMC 1241698. PMID 14527857.
- ^ a b Wilhelms, KW; Kraus, GA; Schroeder, JD; Kim, JW; Cutler, SA; Rasmussen, MA; Anderson, LL; Scanes, CG (2006). "Evaluation of corn furan fatty acid putative endocrine disruptors on reproductive performance in adult female chickens". Poult Sci. 85 (10): 1795–7. doi:10.1093/ps/85.10.1795. PMID 17012171.
- ^ Morris, L. J.; et al. (1966). "A Unique Furanoid Fatty Acid from Exocarpus Seed Oil". Tetrahedron Letters. 7 (36): 4249–4253. doi:10.1016/S0040-4039(00)76045-X.
- ^ Gunstone, F. D.; et al. (1976). "Relative Enrichment of Furan-containing Fatty Acids in the Liver of Starving Cod". Journal of the Chemical Society, Chemical Communications. 16 (16): 630–631. doi:10.1039/C3976000630B.
- ^ Glass, R. L.; Krick, T. P.; Eckhardt, A. E. (1974). "New series of fatty acids in northern pike (Esox lucius)". Lipids. 9 (12): 1004–1008. doi:10.1007/BF02533826. PMID 4444420. S2CID 4052146.