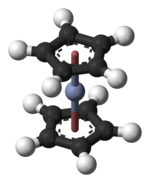
Chromocene is the organochromium compound with the formula [Cr(C5H5)2]. Like structurally related metallocenes, chromocene readily sublimes in a vacuum and is soluble in non-polar organic solvents. It is more formally known as bis(η5-cyclopentadienyl)chromium(II).[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Bis(η5-cyclopentadienyl)chromium(II)
| |||
| Other names
Dicyclopentadienylchromium(II)
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.670 | ||
| EC Number |
| ||
| 3366 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1325 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H10Cr | |||
| Molar mass | 182.186 g·mol−1 | ||
| Appearance | dark red crystals | ||
| Density | 1.43 g/cm3 | ||
| Melting point | 168 to 170 °C (334 to 338 °F; 441 to 443 K) | ||
| Boiling point | Sublimes (under vacuum) | ||
| Insoluble | |||
| Structure | |||
| Pseudooctahedral see Ferrocene | |||
| 0 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Pyrophoric | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H302, H312, H314, H315, H317, H319, H332, H335 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Fe(C5H5)2 Ni(C5H5)2 bis(benzene)chromium chromium(II) acetate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Synthesis
editErnst Otto Fischer, who shared the 1973 Nobel Prize in Chemistry for work on sandwich compounds,[2] first described the synthesis of chromocene.[3][4] One simple method of preparation involves the reaction of chromium(II) chloride with sodium cyclopentadienide:
- CrCl2 + 2 NaC5H5 → Cr(C5H5)2 + 2 NaCl
Such syntheses are typically conducted in tetrahydrofuran. Decamethylchromocene, Cr[C5(CH3)5]2, can be prepared analogously from LiC5(CH3)5. Chromocene can also be prepared from chromium(III) chloride in a redox process:[5]
- 2 CrCl3 + 6 NaC5H5 → 2 Cr(C5H5)2 + C10H10 + 6 NaCl
Structure and bonding
editThe structure of chromocene has been verified by X-ray crystallography. The average Cr–C bond length is 215.1(13) pm.[6] Each molecule contains an atom of chromium bound between two planar systems of five carbon atoms known as cyclopentadienyl (Cp) rings in a sandwich arrangement, which is the reason its formula is often abbreviated as Cp2Cr. Chromocene is structurally similar to ferrocene, the prototype for the metallocene class of compounds. Electron diffraction studies suggest that the Cp rings in chromocene are eclipsed (point group D5h) rather than staggered (point group D5d), though the energy barrier to rotation is small.[7]
With only 16 valence electrons, it does not follow the 18-electron rule.[8] It is a paramagnetic compound.
Reactions
editThe main reactivity associated with chromocene follow from it being highly reducing and the lability of the Cp ligands.
The complex exhibits diverse reactions, usually involving displacement of one cyclopentadienyl ring. Carbonylation has been examined in detail, leads ultimately to chromium hexacarbonyl. An intermediate is cyclopentadienylchromium tricarbonyl dimer:[9]
- 2 Cr(C5H5)2 + 6 CO → [Cr(C5H5)(CO)3]2 + "(C5H5)2"
Chromocene provides a convenient route for preparing the anhydrous form of chromium(II) acetate,[10] a useful precursor to other chromium(II) compounds. The reaction involves the displacement of cyclopentadienyl ligands by the formation of cyclopentadiene:
- 4 CH3CO2H + 2 Cr(C5H5)2 → Cr2(O2CCH3)4 + 4 C5H6
Chromocene decomposes on contact with silica gel to give the Union Carbide catalyst for ethylene polymerization, although other synthetic routes exist for the formation of this important catalyst.
Safety
editChromocene is highly reactive toward air and could ignite upon exposure to the atmosphere.
References
edit- ^ Crabtree, R. H. (2009). The Organometallic Chemistry of the Transition Metals (5th ed.). Hoboken, NJ: John Wiley and Sons. p. 2. ISBN 978-0-470-25762-3.
- ^ "The Nobel Prize in Chemistry 1973". Nobel Foundation. Retrieved 3 December 2012.
- ^ Fischer, E. O.; Hafner, W. (1953). "Di-cyclopentadienyl-chrom". Z. Naturforsch. B (in German). 8 (8): 444–445. doi:10.1515/znb-1953-0809. S2CID 98255073.
- ^ Fischer, E. O.; Hafner, W. (1955). "Cyclopentadienyl-Chrom-Tricarbonyl-Wasserstoff". Z. Naturforsch. B (in German). 10 (3): 140–143. doi:10.1515/znb-1955-0303.
- ^ Long, N. J. (1998). Metallocenes: Introduction to Sandwich Complexes. London: Wiley-Blackwell. ISBN 978-0632041626.
- ^ Flower, K. R.; Hitchcock, P. B. (1996). "Crystal and Molecular Structure of Chromocene (η5-C5H5)2Cr". J. Organomet. Chem. 507 (1–2): 275–277. doi:10.1016/0022-328X(95)05747-D.
- ^ Davis, R.; Kane-Maguire, L.A.P. (1982), "Chromium Compounds with η2–η8 Carbon Ligands", Comprehensive Organometallic Chemistry, Elsevier, pp. 953–1077, doi:10.1016/b978-008046518-0.00041-6, ISBN 978-0-08-046518-0, retrieved 2023-03-26
- ^ Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Concise Introduction (2nd ed.). Wiley-VCH: Weinheim. ISBN 3-527-28165-7.
- ^ Kalousová, Jaroslava; Holeček, Jaroslav; Votinský, Jiři; Beneš, Ludvík (2010). "Das Reaktionsverhalten von Chromocen". Zeitschrift für Chemie. 23 (9): 327–331. doi:10.1002/zfch.19830230903.
- ^ Beneš, L.; Kalousová, J.; Votinský, J. (1985). "Reaction of chromocene with carboxylic acids and some derivatives of acetic acid". J. Organomet. Chem. 290 (2): 147–151. doi:10.1016/0022-328X(85)87428-3.