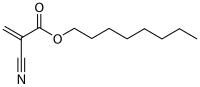
Octyl cyanoacrylate (OCA), a cyanoacrylate ester, is an octyl ester of 2-cyano-2-propenoic acid. It is a clear colorless liquid with a sharp, irritating odor. Its chief use is as the main component of medical cyanoacrylate glues.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Octyl 2-cyanoprop-2-enoate | |
| Other names
Octyl 2-cyanopropenoate
Octyl 2-cyanoacrylate Ocrylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.027.045 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H19NO2 | |
| Molar mass | 209.289 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.0±0.1 g/cm3 |
| Boiling point | 297.6±23.0 °C |
| Reacts | |
| Hazards | |
| Flash point | 137.2±9.4 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In medical and veterinary applications, OCA, n-butyl cyanoacrylate (n-BCA) and isobutyl cyanoacrylate (ICA) are commonly used. They provide rapid wound closure,[1] are bacteriostatic, and their use is usually painless.[citation needed] Butyl esters provide a stronger bond, but the glue is rigid. The octyl ester, while providing weaker bond, is more flexible. Blends of OCA and n-BCA are available which offer both flexibility and a strong bond.
It polymerizes rapidly in presence of moisture.
Heating to higher temperatures causes pyrolysis and depolymerization of the cured glue, producing gaseous products strongly irritating to the lungs and eyes.
References edit
- ^ Singer, A. J.; McClain, S. A.; Katz, A. (2004). "A porcine epistaxis model: hemostatic effects of octylcyanoacrylate". Otolaryngology–Head and Neck Surgery. 130 (5): 553–557. doi:10.1016/j.otohns.2003.09.035. PMID 15138419.