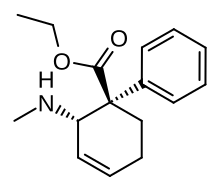
Nortilidine[1] is the major active metabolite of tilidine. It is formed from tilidine by demethylation in the liver. The racemate has opioid analgesic effects roughly equivalent in potency to that of morphine.[2] The (1R,2S) isomer has NMDA antagonist activity. The drug also acts as a dopamine reuptake inhibitor.[3] The reversed-ester of nortilidine is also known, as is the corresponding analogue with the cyclohexene ring replaced by cyclopentane,[4] which have almost identical properties to nortilidine.[5]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H21NO2 |
| Molar mass | 259.349 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Use edit
Nortilidine has been sold as a designer drug, first being identified in Poland in May 2020.[6][7]
See also edit
- Desmetramadol, another opioid metabolite with additional (non-opioid) mechanisms of analgesia, which has also been sold as a designer drug
- Tapentadol
References edit
- ^ US 3792080, "Process for Substituted Cyclohexenes its Products"
- ^ Hajda JP, Jähnchen E, Oie S, Trenk D (November 2002). "Sequential first-pass metabolism of nortilidine: the active metabolite of the synthetic opioid drug tilidine". Journal of Clinical Pharmacology. 42 (11): 1257–61. doi:10.1177/009127002762491352. PMID 12412825. S2CID 12390653.
- ^ Schifano F, Orsolini L, Duccio Papanti G, Corkery JM (February 2015). "Novel psychoactive substances of interest for psychiatry". World Psychiatry. 14 (1): 15–26. doi:10.1002/wps.20174. PMC 4329884. PMID 25655145.
- ^ US 4291059, "Cycloaromatic compounds, analgesic Properties thereof and Method of use thereof as analgesic"
- ^ Personal Communication with Derek P. Reynolds
- ^ "EU Early Warning System Situation Report. Situation report 2". European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). 10 August 2020.
{{cite web}}: Missing or empty|url=(help) - ^ Catalani V, Arillotta D, Corkery JM, Guirguis A, Vento A, Schifano F (2020). "Identifying New/Emerging Psychoactive Substances at the Time of COVID-19; A Web-Based Approach". Frontiers in Psychiatry. 11: 632405. doi:10.3389/fpsyt.2020.632405. PMC 7900492. PMID 33633599.
External links edit
- Media related to Nortilidine at Wikimedia Commons