This discussion of the dental amalgam controversy outlines the debate over whether dental amalgam (the mercury alloy in dental fillings) should be used. Supporters claim that it is safe, effective and long-lasting, while critics argue that amalgam is unsafe because it may cause mercury poisoning and other toxicity.[1][2][3]
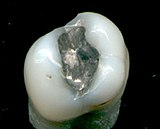
Supporters of amalgam fillings point out that it is safe, durable,[4] relatively inexpensive, and easy to use.[5] On average, amalgam lasts twice as long as resin composites, takes less time to place, is tolerant of saliva or blood contamination during placement (unlike composites), and is often about 20–30% less expensive.[6] Consumer Reports has suggested that many who claim dental amalgam is not safe are "prospecting for disease" and using pseudoscience to scare patients into more lucrative treatment options.[7]
Those opposed to amalgam use suggest that modern composites are improving in strength.[8] In addition to their claims of possible health and ethical issues, opponents of dental amalgam fillings claim amalgam fillings contribute to mercury contamination of the environment. The World Health Organization (WHO) reports that health care facilities, including dental offices, account for as much as 5% of total waste water mercury emissions.[9] The WHO also points out that amalgam separators, installed in the waste water lines of many dental offices, dramatically decrease the release of mercury into the public sewer system.[9] In the United States, most dental practices are prohibited from disposing amalgam waste down the drain.[10] Critics also point to cremation of dental fillings as an additional source of air pollution, contributing about 1% of total global emissions.[11]
The World Health Organization recommends a global phase out of dental mercury in their 2009 report on "Future Use of Materials For Dental Restorations, on the basis of aiming for a general reduction of the use of mercury in all sectors, and on the basis of environmental impacts of mercury product production."[12]
It is the position of the FDI World Dental Federation[13] as well as numerous dental associations and dental public health agencies worldwide[14][15][16][17][18][19][20] that amalgam restorations are safe and effective. Numerous other organizations have also publicly declared the safety and effectiveness of amalgam. These include the Mayo Clinic,[21] Health Canada,[22] Alzheimer's Association,[23] American Academy of Pediatrics,[24] Autism Society of America,[25] U.S. Environmental Protection Agency (EPA),[26] National Multiple Sclerosis Society,[27] New England Journal of Medicine,[28] International Journal of Dentistry,[29] National Council Against Health Fraud,[30] The National Institute of Dental and Craniofacial Research NIDCR,[31] American Cancer Society,[32] Lupus Foundation of America,[33] the American College of Medical Toxicology,[34] the American Academy of Clinical Toxicology,[34] Consumer Reports[7] Prevention,[35] WebMD[36] and the International Association for Dental Research.[37]
The U.S. Food and Drug Administration (FDA) formerly stated that amalgam is "safe for adults and children ages 6 and above"[38] but now recommends against amalgam for children, pregnant/nursing women, and other high-risk groups.[39]
History edit
Dental amalgam has had a long history and global impact.[3] It was first introduced in the Chinese materia medica of Su Kung in 659 A.D. during the Tang dynasty.[3] In Europe, Johannes Stockerus, a municipal physician in Ulm, Germany, recommended amalgam as a filling material as early as 1528.[3] In 1818, Parisian physician Louis Nicolas Regnart added one-tenth by weight of mercury to the fusable metals used as fillings at the time to create a temporarily soft metal alloy at room temperature. Thus, amalgam (an alloy of mercury with another metal or metals, from the French word amalgame) was invented. This was further perfected in 1826, when Auguste Taveau of Paris used a silver paste made from mixing French silver-tin coins with mercury, which offered more plasticity and a quicker setting time.[3] In Europe, prior to 1818, carious teeth were either filled with a melted metal, usually gold or silver (which would often lead to death of the nerve of the tooth from thermal trauma), or the tooth would be extracted.[3]
Further developments edit
In 1855, Dr. J. Foster Flagg, a professor of dental pathology in Philadelphia, experimented with new mixtures of amalgam. In 1861, he presented his findings to the Pennsylvania Association of Dental Surgeons and, in 1881, he published his book, Plastic and Plastic Fillings. (Amalgam fillings were often called "plastic fillings" at the time.) The inevitable result of this affair was that silver amalgam was proven to be "an excellent filling material", and expanded dentistry's "ability to save teeth". Around the same time, John and Charles Tomes in England conducted research on the expansion and contraction of the various amalgam products. During the American Civil War, the debate on the merits of amalgam continued. In dental meetings, with now decades of use and dental research came the recognition of the importance of good technique and proper mixture on long-term success. It was argued, "the fault was not in the material but in the manipulation.... Some men's amalgam is good universally, and some men's gold is bad universally; the difference lies in the preparation of the tooth and in the plug (filling)."[40]
More controversy came in 1872, when an amalgam filling was reported as the cause of death of a Nebraska middle-aged man, resulting in a public outcry against the use of amalgam.[41] His physicians reported that the filling caused swelling of his mouth, throat and windpipe, completely hindering respiration. Given that the involved tooth was a lower second molar, it was later considered very likely that the patient died from Ludwig's Angina, which is a type of cellulitis, rather than mercury poisoning. Another alleged case of "pytalism" causing headache, fever, rapid pulse, metallic taste, loss of appetite, and generalized malaise was reported in 1872 in a female patient following the insertion of eight amalgam fillings.[42] Later, however, another dentist examined the fillings and noted they had, in a short period of time, washed away, and that upon gentle pressure the metal crumbled away. He removed all the fillings with an explorer in three minutes; and concluded poor workmanship alone could have explained the patient's symptoms.
Alfred Stock was a German chemist who reported becoming very ill in the 1920s and traced his illness to his amalgam fillings and resulting mercury intoxication. He described his recovery after the fillings were removed and believed that amalgam fillings would come to be seen as a "sin against humanity".[43] Stock had also previously been exposed to toxic levels of mercury vapor during the course of his work, due to his use of liquid mercury in some novel laboratory apparatus he invented.[44]
1990s to present edit
In the 1990s, several governments evaluated the effects of dental amalgam and concluded that the most likely health effects would be due to hypersensitivity or allergy. Germany, Austria, and Canada recommended against placing amalgam in certain individuals such as pregnant women, children, those with renal dysfunction, and those with an allergy to metals. In 2004, the Life Sciences Research Office analyzed studies related to dental amalgam published after 1996 and concluded that mean urinary mercury concentration (μg of Hg/L in urine, HgU) was the most reliable estimate of mercury exposure.[45] It found that those with dental amalgam were unlikely to reach the levels where adverse effects are seen from occupational exposure (35 μg HgU). Some 95% of study participants had μg HgU below 4–5. Chewing gum, particularly for nicotine, along with more amalgam, seemed to pose the greatest risk of increasing exposure. One gum-chewer had 24.8 μg HgU. Studies have shown that the amount of mercury released during normal chewing is extremely low. It concluded that there was not enough evidence to support or refute many of the other claims such as increased risk of autoimmune disorders, but stated that the broad and nonspecific illness attributed to dental amalgam is not supported by the data.[45] Mutter in Germany, however, concludes, "removal of dental amalgam leads to permanent improvement of various chronic complaints in a relevant number of patients in various trials."[46]
Hal Huggins, a Colorado dentist (previous to having his license revoked), was a notable critic of dental amalgams and other dental therapies he believed to be harmful.[47] His views on amalgam toxicity were featured on 60 Minutes[48] and he was later criticized as a dentist, "prospecting for disease" and having only an "aura of science" by Consumer Reports.[7] In 1996, a Colorado state judge recommended that Huggins's dental license be revoked, for tricking chronically ill patients into thinking that the true cause of their illness was mercury. Time reported the judge's conclusion that Huggins, "diagnosed 'mercury toxicity' in all his patients, including some without amalgam fillings."[49] Huggins's license was subsequently revoked by the Colorado State Board of Dental Examiners for gross negligence and the use of unnecessary and unproven procedures.[50][51][52]
Mercury exposure edit
According to the WHO, all humans are exposed to some level of mercury.[53] Factors that determine whether health effects occur and their severity include the type of mercury concerned (methylmercury and ethylmercury, commonly found in fish, being more serious than elemental mercury); the dose; the age or developmental stage of the person exposed (the foetus is most susceptible); the duration of exposure; and the route of exposure (inhalation, ingestion or dermal contact).[53] The universal standard for examining mercury toxicity is usually discussed in terms of the amount of mercury in the bloodstream for short-term exposure or the amount of mercury excreted in the urine relative to creatine for long-term mercury exposure.[7] Elemental mercury (which is a component of amalgam) is absorbed very differently than methylmercury (which is found in fish).[2] The exposure to mercury from amalgam restorations depends on the number and size of restorations, composition, chewing habits, food texture, grinding, brushing of teeth, and many other physiological factors.[2]
The greatest degree of mercury exposure occurs during filling placement and removal. However, this is not the only time mercury vapors are released. When chewing for extended periods of time (more than 30 minutes) an increased level of mercury vapor is released. Vapor levels will return to normal approximately 90 minutes following chewing cessation. This contributes to a daily mercury exposure for those with amalgam filling.[54]
According to one dental textbook, eating seafood once a week raises urine mercury levels to 5 to 20 µg/L, which is equivalent to two to eight times the level of exposure that comes from numerous amalgam fillings. Neither exposure has any known health effect.[55] Scientists agree that dental amalgam fillings release elemental mercury vapor, but studies report different amounts. Estimates range from 1 to 3 micrograms (µg) per day according to the FDA.[56] The effects of that amount of exposure are also disputed.[45][46]
Newer studies sometimes use mercury vapor analysis instead of the standard exposure test. Because this test was designed for factories and large enclosures, Consumer Reports has reported that this is not an accurate method of analysis for the mouth. It is less reliable, less consistent, and tends to greatly exaggerate the amount of mercury inhaled.[7] Moreover, it is argued this test additionally exaggerates the amount of mercury inhaled by assuming that all the mercury vapor released is inhaled. This assumption was reviewed by the U.S. Department of Health and Human Services and not found to be valid. Their research review found that most of the mercury vapor released from amalgam fillings is mixed with saliva and swallowed, some part is exhaled, and the remaining fraction is inhaled.[57] Of these amounts, it is important to note that the lungs absorb about 80% of inhaled mercury.[57]
A study conducted by measuring the intraoral vapour levels over a 24-hour period in patients with at least nine amalgam restorations showed that the average daily dose of inhaled mercury vapour was 1.7 μg (range from 0.4 to 4.4 μg), which is approximately 1% of the threshold limit value of 300 to 500 μg/day established by the WHO, based on a maximum allowable environmental level of 50 μg/day in the workplace.[2] Critics point out that: (1) the workplace safety standards are based on allowable maxima in the workplace, not mercury body burden; (2) the workplace safety numbers are not applicable to continuous 24-hour exposure, but are limited to a normal work day and a 40-hour workweek;[58] and (3) the uptake/absorption numbers are averages and not worst-case patients (those most at risk).[59]
A test that was done throughout the 1980s by some opposition groups and holistic dentists was the skin patch test for mercury allergies. As part of "prospecting for disease", Consumer Reports wrote that these groups had placed high doses of mercuric chloride on a skin patch which was guaranteed to produce irritation on the patient's skin and subsequent revenue for the person administering the test.[7]
The current recommendations for residential exposure (not including amalgam fillings already accounted for) are as follows: The ATSDR Action Level for indoor mercury vapor in residential settings is 1 µg/m3 and the ATSDR MRL (Minimal Risk Level) for chronic exposure is 0.2 µg/m3[60] According to the ATSDR, the MRL(Minimal Risk Level) is an estimate of the level of daily exposure to a substance that is unlikely to cause adverse non-cancerous health effects. The Action Level is defined as an indoor air concentration of mercury that would prompt officials to consider implementing response actions. It is a recommendation and does not necessarily imply toxicity or health risks.[60] Breathing air with a concentration of 0.2 µg mercury/m3 would lead to an inhaled amount of approximately 4 µg/day (respiratory volume of 20m3/day). About 80% of inhaled mercury vapor would be absorbed.[61]
A 2003 monograph on mercury toxicity from the WHO concluded that dental amalgam contributes significantly to mercury body burden in humans with amalgam fillings and that dental amalgam is the most common form of exposure to elemental mercury in the general population, constituting a potentially significant source of exposure to elemental mercury. Estimates of daily intake from amalgam restorations range from 1 to 12.5 μg/day, with the majority of dental amalgam holders being exposed to less than 5 μg mercury/day.[61] They also note that this will continue to decline as the number of amalgam restorations is declining.
Health research edit
As public pressure demands more research on amalgam safety, an increasing number of studies with larger sample sizes are being conducted. Those who are not opposed to amalgam claim that, aside from rare and localized tissue irritation, recent evidence-based research has continued to demonstrate no ill effects from the minute amounts of mercury exposure from amalgam fillings.[14][62][63] A 2004 systematic review conducted by the Life Sciences Research Office, whose clients include the FDA and NIH, concluded, "the current data are insufficient to support an association between mercury release from dental amalgam and the various complaints that have been attributed to this restoration material."[45] A systematic review in 2009 demonstrated that mercury released from amalgam restorations does not give rise to toxic effects on the nervous system of children.[64] In 2014, a Cochrane Systematic review found "insufficient evidence to support or refute any adverse effects associated with amalgam or composite restorations."[65]
Those opposed to dental amalgam suggest that mercury from dental amalgam may lead to nephrotoxicity, neurobehavioural changes, autoimmunity, oxidative stress, autism, skin and mucosa alterations, non-specific symptoms and complaints, Alzheimer's disease, calcium-building in the kidneys, kidney stones, thyroid issues, and multiple sclerosis.[46]
Autoimmune disorders edit
Both those opposed and those not opposed to dental amalgam recognize that amalgam has been found to be a rare contributor to localized and temporary tissue irritation known as oral lichenoid lesions.[14][62][63][66] These mild, lichenoid reactions have also been reported in composite resin fillings.[67] Those opposed to amalgam believe that amalgam fillings are also associated with increased risk of other autoimmune conditions such as multiple sclerosis (MS), lupus, thyroiditis, and eczema.[68]
Consumer Reports considered these alleged associations between amalgam and chronic disease made by some health practitioners as "prospecting for diseases".[7] The National Multiple Sclerosis Society (USA) similarly has stated, "There is no scientific evidence to connect the development or worsening of MS with dental fillings containing mercury, and therefore no reason to have those fillings removed. Although poisoning with heavy metals-such as mercury, lead, or manganese can damage the nervous system and produce symptoms such as tremor and weakness, the damage is inflicted in a different way than occurs in MS and the process is also different."[27] The Lupus Foundation of America also states on their website, "At the present time, we do not have any scientific data that indicates that dental fillings may act as a trigger of lupus. In fact, it is highly unlikely that dental fillings aggravate or cause SLE."[33]
Dental staff impact edit
In 2006, a literature review was undertaken to evaluate the research on amalgam and its potential health effects on dentists and dental staff.[69] It was reported that there is currently no conclusive epidemiological evidence regarding risks for adverse reproductive outcomes associated with mercury and dental professionals. It is mentioned that evidence to date fails to account for all confounding variables (such as alcohol consumption) and recommends more comprehensive and rigorous studies to adequately assess the hazards faced by dental personnel.[69]
Removal of amalgam edit
The American College of Medical Toxicology and the American Academy of Clinical Toxicology still claim that mercury from amalgams does not cause illness because "the amount of mercury that they release is not enough to cause a health problem".[34] In response to some people wanting their existing amalgam removed for fear of mercury poisoning, these societies advise that the removal of fillings is likely to cause a greater exposure to mercury than leaving the fillings in place.[34] These societies also claim that removal of amalgam fillings, in addition to being unnecessary health care and likely to cause more mercury exposure than leaving them in place, is also expensive.[34]
Dentists who advocate removal of amalgam fillings often recommend wearing breathing apparatus, using high-volume aspiration, and performing the procedure as quickly as possible. Sources of mercury from the diet, and the potential harm of the composite resins to replace the purportedly harmful amalgam fillings, may also need to be considered.[70]
Alternative materials edit
Alternative materials which may be suitable in some situations include composite resins, glass ionomer cements, porcelain and gold alloys.[71] Most of these materials, with the notable exception of gold, have not been used as long as amalgam, and some are known to contain other potentially hazardous compounds. Teaching of amalgam techniques to dental students is declining in some schools in favor of composite resin,[72] and at least one school, University of Nijmegen in the Netherlands, eliminated dental amalgam from the curriculum entirely in 2001.[73] This is largely a response to consumer pressure for white fillings for cosmetic reasons, and also because of the increasing longevity of modern resin composites. These alternative dental restorative materials are not free of potential health risks, such as allergenicity, inhalation of resin dust, cytotoxicity, and retinal damage from blue curing light.[74]
Chelation therapy edit
Anti-amalgam sources typically promote removal of amalgam fillings and substitution with other materials. Detoxification may also be advised, including fasting, restricted dieting to avoid mercury containing foods, and quasi-chelation therapies, allegedly to remove accumulated mercury from the body.[75] The American College of Medical Toxicology and the American Academy of Clinical Toxicology recommend against chelation therapy and say that chelation therapy can artificially and temporarily elevate the levels of heavy metals in the urine (a practice referred to as "provoked" urine testing).[34] They also mention that the chelating drugs may have significant side effects, including dehydration, hypocalcemia, kidney injury, liver enzyme elevations, hypotension, allergic reactions, and mineral deficiencies.[34]
Epidemiology edit
Better dental health overall coupled with increased demand for more modern alternatives such as resin composite fillings (which match the tooth color), as well as public concern about the mercury content of dental amalgam, have resulted in a steady decline in dental amalgam use[76] in developed countries, though overall amalgam use continues to rise worldwide. Given its superior strength, durability, and long life relative to the more expensive composite fillings, it will likely be used for many years to come.[77][78] Over a lifetime, dietary sources of mercury are far higher than would ever be received from the presence of amalgam fillings in the mouth. For example, due to pollution of the world's oceans with heavy metals, products such as cod liver oil may contain significant levels of mercury.
Prenatal edit
There is little evidence to suggest that amalgam fillings have any negative direct effects on pregnancy outcomes or on an infant post-pregnancy. A study, consisting of 72 pregnant women, was conducted to determine the effects of dental amalgam on fetuses in utero. Results indicated that although the amount of amalgam the mother had was directly related to the amount of mercury in the amniotic fluid, no negative effects on the fetus were found. A larger study, consisting of 5,585 women who had recently given birth, was used to determine if amalgam restorations during pregnancy had any effects on infant birthweight. Among the study group, 1,117 women had infants with low birth weights and 4,468 women had infants with normal birth weights. Approximately five percent of the women had one or more amalgam filling restorations during their pregnancy term. These women had little to no difference in infant birth weight compared to the women whom did not undergo amalgam restoration during pregnancy.[2]
Public awareness edit
A 2006 Zogby International poll of 2,590 US adults found that 72% of respondents were not aware that mercury was a main component of dental amalgam and 92% of respondents would prefer to be told about mercury in dental amalgam before receiving it as a filling.[79] A 1993 study published in FDA Consumer found that 50% of Americans believed fillings containing mercury caused health problems.[80] Some dentists (including a member of the FDA's Dental Products Panel) suggest that there is an obligation to inform patients that amalgam contains mercury.[81][82]
A prominent debate occurred in the late 20th century, with consumer and regulatory pressure to eliminate amalgam being "at an all-time high".[82] In a 2006 nationwide poll, 76% of Americans were unaware that mercury is the primary component in amalgam fillings,[79] and this lack of informed consent was the most consistent issue raised in a recent U.S. Food and Drug Administration (FDA) panel on the issue by panel members.[82]
The broad lack of knowledge that existed among the public was also displayed when a December 1990 episode of the CBS news program 60 Minutes covered mercury in amalgam. This resulted in a nationwide amalgam scare and additional research into mercury release from amalgam. The following month Consumer Reports published an article criticizing the content of the broadcast, stating that it contained a great deal of false information and that the ADA spokesperson on the program was ill-prepared to defend the claims.[7] For example, 60 Minutes reported that Germany was planning to pass legislation within the year to ban amalgam, but the institute of German Dentists said one month later that there was no such law pending. Also, one physiologist interviewed by Consumer Reports noted that the testimonials are mostly anecdotal, and both the reported symptoms and the rapid recovery time after the fillings are removed are physiologically inconsistent with that of mercury poisoning. Consumer Reports goes on to criticize how 60 Minutes failed to interview the many patients who had fillings or teeth removed only to have the symptoms stay the same or get worse.[7]
In 1991, the United States Food and Drug Administration concluded, "none of the data presented show a direct hazard to humans from dental amalgams."[83] In 2002, a class action lawsuit was initiated by patients who felt their amalgam fillings caused them harm. The lawsuit named the ADA, the New York Dental Association, and the Fifth District Dental Society for deceiving "[the] public about health risks allegedly associated with dental amalgam." On 18 February 2003, the New York Supreme Court dismissed the two amalgam-related lawsuits against organized dentistry, stating the plaintiffs had "failed to show a 'cognizable cause of action'".[84]
Research directions edit
The proper interpretation of the data is considered controversial only by those opposed to amalgam. The vast majority of past studies have concluded that amalgams are safe. However, although the vast majority of patients with amalgam fillings are exposed to levels too low to pose a risk to health, many patients (i.e. those in top 0.1%) exhibit urine test results which are comparable to the maximum allowable legal limits for long-term work place (occupational) safety.[58][59] Two recent randomized clinical trials in children[85] discovered no statistically significant differences in adverse neuropsychological or renal effects observed over a five-year period in children whose caries were restored using dental amalgam or composite materials. In contrast, one study showed a trend of higher dental treatment need later in children with composite dental fillings, and thus claimed that amalgam fillings are more durable. However, the other study (published in JAMA) cites increased mercury blood levels in children with amalgam fillings. The study states, "during follow-up [blood mercury levels were] 1.0 to 1.5 μg higher in the amalgam group than in the composite group." EPA considers high blood mercury levels to be harmful to the fetus and also states, "exposure at high levels can harm the brain, heart, kidneys, lungs, and immune system of people of all ages." Currently, EPA has set the "safe" mercury exposure level to be at 5.8 μg of mercury per one liter of blood.[86] While mercury fillings themselves do not increase mercury levels above "safe" levels, they have been shown to contribute to such increase. However, such studies were unable to find any negative neurobehavioral effects.[87][86][88]
Environmental impact edit
Environmental concerns over external costs exist as well.[89] In the United States, dental amalgam is the largest source of mercury received by sewage treatment plants. The mercury contaminates the treatment plant sludge, which is typically disposed of by land application, landfilling or incineration.[10] In the United States, several states, including New Jersey,[90] New York,[91] and Michigan,[92] required the installation of dental amalgam separators prior to 2017.[93] EPA promulgated an effluent guidelines regulation in 2017 which prohibits most dental practices from disposing amalgam waste down the drain. Most dental offices nationwide are now required to use amalgam separators.[10][94]
The WHO reported in 2005 that in the United Kingdom mercury from amalgam accounted for 5% of total mercury emissions.[9] In Canada, dental amalgam was estimated to contribute one-third of the mercury in sewer system waste, but it is believed amalgam separators in dental offices may dramatically decrease this burden on the public sewer system.[9]
The 2005 WHO report stated that mercury from amalgam was approximately 1% of total global mercury emissions, and that one-third of the total mercury in most sewage systems was discharged from dental offices.[9] Other studies have shown this to be a gross exaggeration or not reflective of developed countries. With respect to pollution in the United States, a study done in 1992 showed that batteries "accounted for 86 percent of discarded mercury and dental amalgam a mere 0.56 percent".[95] Mercury is an environmental contaminant and the WHO, OSHA, and NIOSH have established specific occupational exposure limits. Mercury imposes health risks upon the surrounding population. In economics, this pollution is considered an external cost not factored into the private costs of using mercury-based products. Environmental risks from amalgam can be mitigated by amalgam separators and the ISO has issued standards regarding the proper handling and disposal of amalgam waste.[96] Mercury is a naturally occurring element that is present throughout the environment[97][98] and the vast majority of the pollution (about 99%) comes from large-scale human industrial activity (such as coal-fired electricity generation, hydroelectric dams, and mining, which increase both airborne and waterborne mercury levels).[98][99] Eventually, the airborne mercury finds its way into lakes, rivers, and oceans, where it is consumed by aquatic life.[98] Amalgam separators may dramatically decrease the release of mercury into the public sewer system, but they are not mandatory in some jurisdictions.[100] When mercury from these sources enters bodies of water, especially acidic bodies of water, it can be converted into the more toxic methylmercury.[101]
Cremation of bodies containing amalgam restorations results in near-complete emission of the mercury into the atmosphere, as the temperature in cremation is far greater than the boiling point of mercury. In countries with high cremation rates (such as the UK), mercury has become a great concern. Proposals to remedy the situation have ranged from removing amalgam-containing teeth prior to cremation to installing activated carbon adsorption or other post-combustion mercury capture technology in the flue gas stream. According to the United Nations Environment Programme, it is estimated that globally about 3.6 tonnes of mercury vapor was emitted into the air through cremation in 2010, or about 1% of total global emissions.[11] Mercury emissions from cremation are growing in the US, both because cremation rates are increasing and because the number of teeth in the deceased are increasing due to better dental care.[citation needed] Since amalgam restorations are very durable and relatively inexpensive, many of the older deceased have amalgam restorations.[citation needed] According to work done in Great Britain,[citation needed] mercury emissions from cremation are expected to increase until at least 2020.
Organizational statements edit
American Dental Association (ADA) edit
The American Dental Association (ADA) has asserted that dental amalgam is safe and has held, "the removal of amalgam restorations from the non-allergic patient for the alleged purpose of removing toxic substances from the body, when such treatment is performed solely at the recommendation or suggestion of the dentist, is improper and unethical".[102] Under the comments of the American Dental Association before the FDA's Dental Products Panel[103] of the Medical Devices Advisory Committee,[104] the ADA supports the 2009 FDA ruling on dental amalgam.[14][105] ADA states, "dental amalgam has an established record of safety and effectiveness, which the scientific community has extensively reviewed and affirmed."[106][107][108] The ADA also supports the 2017 EPA wastewater regulation and is providing information and assistance to its members in implementation of amalgam separators.[109] The ADA asserts the best scientific evidence supports the safety of dental amalgam.[110] Clinical studies have not established an occasional connection between dental amalgam and adverse health effects in the general population.[111]
Dental Material Commission edit
In 2002, Dr. Maths Berlin of The Dental Material Commission published an overview and assessment of the scientific literature published between November 1997 and 2002 for the Swedish Government on amalgam and its possible environmental and health risks.[112] A final report was submitted to the Swedish Government in 2003 and included his report as an annex to the full report. In the final report from 2003, Berlin states that the 1997 summary had found "... no known epidemiological population study has demonstrated any adverse health effects in amalgam". He reports that researchers have been able to show effects of mercury at lower concentrations than before and states, "... the safety margin that it was thought existed with respect to mercury exposure from amalgam has been erased." He recommends eliminating amalgam in dentistry for medical and environmental reasons as soon as possible.[112]
Food and Drug Administration edit
After FDA's deliberations and review of hundreds of scientific studies relating to the safety of dental amalgam, the FDA concluded, "clinical studies have not established a causal link between dental amalgam and adverse health effects in adults and children age six and older."[113] The FDA concluded that individuals age six and older are not at risk of mercury-associated health effects from mercury vapor exposure that come from dental amalgam.[105]
In 2009, the FDA issued a final rule which classified dental amalgam as a "Class II" (moderate risk) device, placing it in the same category as composite resins and gold fillings.[14] In a press release announcing the reclassification, the agency again stated, "the levels [of mercury] released by dental amalgam fillings are not high enough to cause harm in patients."[114]
Also, in the FDA final regulation on dental amalgam in 2009, the FDA recommended the product labeling of dental amalgam. The suggested labeling included: a warning against the use of dental amalgam in patients with mercury allergy, a warning that dental professionals use appropriate ventilation when handling dental amalgam, and a statement discussion of scientific evidence on dental amalgam's risks and benefits in order to make informed decisions amongst patient and professional dentists.[105][115]
In 2020, the FDA updated their guidelines to recommend against amalgam for certain high-risk groups, including children, pregnant and nursing women, people with neurological disease, impaired kidney function, and known sensitivity to mercury, due to potential harmful health effects of mercury vapor.[39] They acknowledge that breathing in mercury vapor may be harmful to certain populations, but recommend against removal of amalgam fillings unless medically necessary.[116]
Regulation edit
The use of mercury in dental fillings is considered safe and effective in all countries practicing modern dentistry (see below). There are currently two countries, Norway and Sweden, that have introduced legislation to prohibit or restrict use of amalgam fillings; however, in both cases amalgam is part of a larger program of reducing mercury in the environment and includes the banning of mercury-based batteries, thermometers, light bulbs, sphygmomanometers, consumer electronics, vehicle components, etc. In many countries, unused dental amalgam after a treatment is subject to disposal protocols for environmental reasons. Over 100 countries are signatories to the United Nations "Minamata Convention on Mercury".[117] Unlike mercury-based batteries, cosmetics, and medical devices, which were banned as of the year 2020, the treaty has not banned the use of dental amalgam, but allows phasing down amalgam use over a time period appropriate to domestic needs, an approach advocated by the World Health Organization (WHO).[118][119]
International groups edit
FDI World Dental Federation recognizes the safety and effectiveness of amalgam restorations. FDI is a federation of approximately 200 national dental associations and dental specialist groups representing over 1.5 million dentists. In collaboration with the WHO, they have produced an FDI position statement and WHO consensus statement on dental amalgam.[13] Their position regarding the safety of dental amalgam is that, aside from rare allergic reactions and local side effects, "the small amount of mercury released from amalgam restorations, especially during placement and removal, has not been shown to cause any other adverse health effects." The paper goes on to say that there have been "no controlled studies published that show adverse systemic effects" from amalgam restorations and there is no evidence that removing amalgam restorations relieves any general symptoms. More recently, FDI has published a resolution confirming that their position on the safety and effectiveness of amalgam has not changed despite the phasing-down in some countries.[120]
North America edit
In the United States, numerous respected professional and non-profit organizations consider amalgam use to be safe and effective and have publicly declared such.[5] In addition to the American Dental Association,[14][121] other American organizations, including the Mayo Clinic,[21] the U.S. Food and Drug Administration (FDA),[38] Alzheimer's Association,[23] American Academy of Pediatrics,[24] Autism Society of America,[25] U.S. Environmental Protection Agency,[26] National Multiple Sclerosis Society,[27] New England Journal of Medicine,[28] International Journal of Dentistry,[29] National Council Against Health Fraud,[30] The National Institute of Dental and Craniofacial Research NIDCR,[31] American Cancer Society,[32] Lupus Foundation of America,[33] Consumer Reports[7] and WebMD[36] have all given formal, public statements declaring that amalgam fillings are safe based on the best scientific evidence.
On 28 July 2009, the U.S. Food and Drug Administration (FDA) recategorized amalgam as a class II medical device, which critics claim indicates a change in their perception of safety. The ADA has indicated that this new regulation actually places encapsulated amalgam in the same class of devices as most other restorative materials, including composite and gold fillings.[14]
Despite the research regarding the safety of amalgam fillings, the state of California requires warning information given to patients for legal reasons (informed consent) as part of Proposition 65. This warning also applied to resin fillings for a time, since they contain bisphenol A (BPA) a chemical known to cause reproductive toxicity at high doses.[122]
In Canada, amalgam use is considered safe and effective by some groups. A 2005 position statement from the Canadian Dental Association (CDA) states "current scientific evidence on the use of dental amalgam supports that amalgam is an effective and safe filling material that provides a long-lasting solution for a broad range of clinical situations. The CDA has established its position based on the current consensus of scientific and clinical experts and on recent extensive reviews of strong evidence by major North American and international organizations, which have satisfactorily countered any safety concerns."[15] Amalgam use is regulated by Health Canada as are all medical treatments[123] and Health Canada has also stated that dental amalgam is not causing illness in the general population.[22]
Australia edit
Australia recognizes the safety and effectiveness of amalgam restorations. In 2012, the Australian Dental Association published a position paper on the safety of dental amalgam.[16] Their position is "Dental Amalgam has been used as a dental restorative material for more than 150 years. It has proved to be a durable, safe and effective material which has been the subject of extensive research over this time" and "amalgam should continue to be available as a dental restorative material".[124]
Europe edit
Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) is a scientific committee within the European Commission. In a 2008 document of 74 pages, its research on the subject of amalgam safety concluded "there is no scientific evidence for risks of adverse systemic effects exist [sic] and the current use of dental amalgam does not pose a risk of systemic disease."[125]
England and Scotland recognize the safety and effectiveness of amalgam restorations. A policy statement from the British Dental Health Foundation states that they do not consider amalgams containing mercury a significant health risk.[126]
Ireland recognizes the safety and effectiveness of amalgam restorations. The Irish Dental Association has published on their website: "Dental amalgam has been used on patients for over 150 years. All available world-wide research indicates that amalgam is not harmful to health.... No Government or reputable scientific, medical or dental body anywhere in the world accepts, on any published evidence, that dental amalgam is a hazard to health."[18] The Irish Dental Association provides additional detail in a published patient information letter.[19]
France has publicly recognized the safety and effectiveness of amalgam restorations. A position paper on the Association Dentaire Française website states that amalgam restorations have proven to have no biological or toxic effect.[20] They also mention that no serious pathological fact has ever been observed and no general toxic action has been scientifically proven to date.[20] The most exposed subjects remain dentists in whom it did not identify occupational diseases related to mercury and other rare that any allergies. These amalgam allergies are about 40 times less than that of resin fillings[20]
During the 1980s and 1990s in Norway, there was considerable and intense public debate on the use of dental amalgam.[127] The Norwegian Dental Patients Association (Forbundet Tenner og Helse), made up of people who believe they suffered health effects from amalgam fillings, was a driving force in this debate.[127] During this time, the media often featured interviews with people claiming that their health problems were caused by amalgam fillings, and who have regained their health after replacing their amalgam fillings with a different material. Some scientific studies also reported that patients have been restored to health after having had their amalgam fillings replaced. However, these studies were heavily disputed at the time and the Norwegian Board of Health still maintain there is no scientifically proven connection between dental amalgam and health problems.[127]
In 1991, organized through the ministry of the environment, Norway began phasing out the use of most mercury-containing products (not limited to amalgam fillings but also including mercury-based batteries, thermometers, sphygmomanometers, consumer electronics, vehicle components, etc.).[128] The ban on the import, export, and use of most mercury-based products began on 1 January 2008.[128] The Norwegian officials stressed that this is not a decision based on using an unsafe health product, but rather that the "overall, long term goal is to eliminate the use and release of mercury to the environment".[127] Despite the mercury ban, dental offices in Norway may apply for exemptions to use amalgam on a case-by-case basis.[127]
Similar to Norway, from 1995 to 2009 the Environment Ministry of the Government of Sweden gradually banned the import and use of all mercury-based products (not limited to amalgam fillings alone, but also including mercury-based batteries, thermometers, sphygmomanometers, consumer electronics, vehicle components, lightbulbs, analytical chemicals, cosmetics, etc.).[129][130] These mercury-based products were phased out for environmental reasons and precautionary health reasons.[131] Like Norway, there was considerable public pressure in the years leading up to the ban.[127] Since the ban, the Government of Sweden continued to investigate ways of reducing mercury pollution.[132] The Swedish Chemicals Agency state that they may grant exemptions on the use of amalgam on a case-by-case basis.[132]
Following the Minamata Convention on Mercury, from July 2018 onwards, the EU Mercury Regulation prohibits the use of dental amalgam in children under 15 years old and pregnant or breastfeeding woman. Additional requirements include the use of pre-encapsulated mercury and ethical disposal of waste amalgam.[133] The British Dental Association has worked with the Council of European Dentists to prevent an immediate ban of amalgam until further research into practicalities has been undertaken,[134] which is currently ongoing.[135][136] The European Commission will report to European Parliament by June 2020, and to the European Council by 2030 regarding the viability of ending dental amalgam use by 2030.[133]
Japan edit
In Japan, the use of amalgam began to decline around the 1990s; since 2016, fillings with amalgam alloys have been excluded from insurance coverage. Amalgam is still allowed as of 2023, but is rarely used because it is very expensive. Dental composite and palladium alloys are used instead.[137]
See also edit
References edit
- ^ American Journal of Dental Science. Massachusetts, U.S: Harvard University; 1845. American Society of Dental Surgeons; p. 170.[title missing]
- ^ a b c d e Rathore M, Singh A, Pant VA (2012). "The dental amalgam toxicity fear: a myth or actuality". Toxicology International. 19 (2): 81–8. doi:10.4103/0971-6580.97191. PMC 3388771. PMID 22778502.
- ^ a b c d e f "Amalgam: Its History and Perils", California Dental Association Journal. March 2006. pages 215-229. 2006.
- ^ Allan, DN (1977). "A longitudinal study of dental restorations". British Dental Journal. 143 (3): 87–9. doi:10.1038/sj.bdj.4803949. PMID 268962. S2CID 4857158.
- ^ a b "Dental Amalgam: What Others Say". American Dental Association. Retrieved 12 June 2015.
- ^ Moffa JP (1989). "Comparative performance of amalgam and composite resin restorations and criteria for their use". In Kenneth J. Anusavice (ed.). Quality evaluation of dental restorations: criteria for placement and replacement : proceedings of the International Symposium on Criteria for Placement and Replacement of Dental Restorations, Lake Buena Vista, Florida, October 19–21, 1987. Carol Stream, Illinois: Quintessence Publishing. pp. 125–38. ISBN 978-0-86715-202-9.
- ^ a b c d e f g h i j "The mercury in your mouth: You can avoid amalgam fillings or even replace the ones you have, but should you?" Consumer Reports 1991. 56:316-319
- ^ Leinfelder, Karl F. (2000). "Do Restorations Made of Amalgam Outlast Those Made of Resin-Based Composite?". The Journal of the American Dental Association. 131 (8): 1186–7. doi:10.14219/jada.archive.2000.0355. PMID 10953536. Archived from the original on 13 July 2012.
- ^ a b c d e "Mercury in Health Care : Policy Paper" (PDF). World Health Organization. August 2005. Retrieved 13 June 2015.
- ^ a b c "Dental Effluent Guidelines". Washington, D.C.: U.S. Environmental Protection Agency (EPA). 18 October 2021.
- ^ a b "Switzerland. United Nations Environment Programme. UNEP Division of Technology, Industry and Economics. Global Mercury Assessment 2013. Geneva, Switzerland: UNEP Chemicals, 2013. PDF" (PDF). Archived from the original (PDF) on 1 April 2014. Retrieved 26 November 2014.
- ^ "Future Use of Materials for Dental Restoration". World Health Organization.
- ^ a b "WHO Consensus Statement on Dental Amalgam" (PDF). FDI. Archived from the original (PDF) on 14 August 2014. Retrieved 13 June 2015.
- ^ a b c d e f g "Statement on Dental Amalgam". American Dental Association. Retrieved 12 June 2015.
- ^ a b "Dental Amalgam" (PDF). Canadian Development Association. Retrieved 13 June 2015.
- ^ a b "Safety of Dental Amalgam" (PDF). American Dental Association. Retrieved 13 June 2015.
- ^ "In the dentist's chair". British Homeopathic Association. 13 June 2013. Retrieved 12 June 2015.
- ^ a b "How safe are Amalgam / Mercury fillings?". Irish Dental Association. Retrieved 12 June 2015.
- ^ a b "Patient Information on Dental Amalgam" (PDF). Irishealth.com. Archived from the original (PDF) on 13 January 2015. Retrieved 13 June 2015.
- ^ a b c d "Amalgames dentaires". Association Dentaire Française. Archived from the original on 13 January 2015. Retrieved 12 June 2015.
- ^ a b "Amalgam is a Safe and Durable Choice for Fillings". Mayo Clinic News Network. 25 January 2013. Retrieved 12 June 2015.
- ^ a b "The Safety of Dental Amalgam". Health Canada. 1996. Retrieved 12 June 2015.
- ^ a b "Memory Loss Myths & Facts". Alzheimer's Association. Retrieved 12 June 2015.
- ^ a b [1] Archived 29 February 2012 at the Wayback Machine
- ^ a b "Causes". Autism Society. Retrieved 12 June 2015.
- ^ a b "Mercury in Dental Amalgam". EPA. 8 July 2013. Archived from the original on 24 September 2015.
- ^ a b c "Complementary & Alternative Medicines". National Multiple Sclerosis Society. Retrieved 12 June 2015.
- ^ a b New England Journal of Medicine 349; 18, 30 October 2003, pp. 1731–1737.[title missing]
- ^ a b Uçar, Y; Brantley, W. A. (2011). "Biocompatibility of Dental Amalgams". International Journal of Dentistry. 2011: 1–7. doi:10.1155/2011/981595. PMC 3227436. PMID 22145006.
- ^ a b "NCAHF Position Paper on Amalgam Fillings". NCAHF. Retrieved 12 June 2015.
- ^ a b "Studies Evaluate Health Effects of Dental Amalgam Fillings in Children". National Institute of Dental and Craniofacial Research. 18 April 2006. Retrieved 12 June 2015.
- ^ a b Complete Guide to Complementary and Alternative Cancer Therapies, 2nd edition, 2009, pp. 164–166.
- ^ a b c "Get Answers: Living well with lupus". Lupus Foundation of America. Archived from the original on 19 April 2013. Retrieved 12 June 2015.
- ^ a b c d e f g "Five Things Physicians and Patients Should Question". Choosing Wisely: an initiative of the ABIM Foundation. American College of Medical Toxicology, American Academy of Clinical Toxicology. February 2013. Retrieved 5 December 2013.
- ^ "Health: Mercury Dental Fillings". Prevention. 3 November 2011. Retrieved 12 June 2015.
- ^ a b "Frequently Asked Questions About Dental Health". Webmd. Retrieved 12 June 2015.
- ^ Ajiboye, A.S.; Mossey, P.A.; Fox, C.H. (1 July 2020). "International Association for Dental Research Policy and Position Statements on the Safety of Dental Amalgam". Journal of Dental Research. 99 (7): 763–768. doi:10.1177/0022034520915878. ISSN 0022-0345. PMID 32315245. S2CID 216072888.
- ^ a b "About Dental Amalgam Fillings". FDA. Archived from the original on 9 March 2020. Retrieved 12 June 2015.
- ^ a b Commissioner, Office of the (24 September 2020). "FDA Issues Recommendations for Certain High-Risk Groups Regarding Mercury-Containing Dental Amalgam". FDA. Retrieved 18 April 2023.
- ^ Hoffmann-Axthelm W, History of dentistry. Trans by Koehler HM. Quintessence, Chicago, 1981 ISBN 9783876521619. pp: 43, 156
- ^ Brown GV I, The surgery of oral diseases and malformations: their diagnosis and treatment. Lea and Febiger, Philadelphia, third ed., p:168, 1918.
- ^ Scott H, "Pytalism from amalgam fillings". Dent Register 26:384–5, 1872
- ^ Stock, Alfred (1926). "Die Gefaehrlichkeit des Quecksilberdampfes" [The Hazards of Mercury Vapor]. Zeitschrift für Angewandte Chemie. 39 (15): 461–466. Bibcode:1926AngCh..39..461S. doi:10.1002/ange.19260391502. Archived from the original on 21 October 2012. Retrieved 25 November 2008.
- ^ Sella, Andrea (20 May 2014). "Stock's valve".
- ^ a b c d Brownawell AM, Berent S, Brent RL, et al. (2005). "The potential adverse health effects of dental amalgam". Toxicol Rev. 24 (1): 1–10. doi:10.2165/00139709-200524010-00001. PMID 16042501. S2CID 24749507.
- ^ a b c Mutter, J; Naumann, J; Walach, H; Daschner, F (2005). "Amalgam: Eine Risikobewertung unter Berücksichtigung der neuen Literatur bis 2005" [Amalgam risk assessment with coverage of references up to 2005]. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) (in German). 67 (3): 204–16. doi:10.1055/s-2005-857962. PMID 15789284. S2CID 72012543.
- ^ Huggins, Hal A.; Anderson (1993). It's All in Your Head: The Link Between Mercury Amalgams and Illness. Avery Publishing. ISBN 978-0-89529-550-7.
- ^ CBS's 60 Minutes, 16 December 1990[title missing]
- ^ Leon Jaroff (8 May 2002). "There's Nothing Dangerous About 'Silver' Fillings". Time. Retrieved 23 January 2015.
- ^ "Case No. DE 95-04 : Initial Decision" (PDF). Center for Inquiry. Archived from the original (PDF) on 21 October 2018. Retrieved 13 June 2015.
- ^ Staudenmayer, Herman (1998). Environmental Illness: Myth and Reality. CRC Press. pp. 400 pages. ISBN 978-1-56670-305-5.
- ^ Radford, Bill (23 February 2003). "Anti-amalgam pioneer no stranger to controversy". The Gazette. Colorado Springs.
- ^ a b "Mercury and health". World Health Organization. Retrieved 12 June 2015.
- ^ Lorscheider, F. (1995). "The Dental Amalgam Mercury Controversy - Inorganic Mercury and the CNS; Genetic Linkage of Mercury and Antibiotic Resistances in Intestinal Bacteria". Toxicology. 97 (1–3): 19–22. doi:10.1016/0300-483X(94)02964-V. PMID 7716785.
- ^ Craig's Restorative Dental Materials, 12th Edition. C.V. Mosby, 2006. page 255
- ^ "Dental Amalgam: Myths vs. Facts" (Press release). American Dental Association. July 2002. Archived from the original on 13 February 2009. Retrieved 23 May 2014.
- ^ a b "Dental Amalgam: A Scientific Review and Recommended Public Health Service Strategy for Research, Education and Regulation Final Report of the Subcommittee on Risk Management of the Committee to Coordinate Environmental Health and Related Programs Appendix III". Department of Health and Human Services, Public Health Service. January 1993. Archived from the original on 7 January 2015. Retrieved 13 January 2015.
- ^ a b http://www.osha.gov/SLTC/healthguidelines/mercuryvapor/recognition.html Archived 13 July 2011 at the Wayback Machine[title missing]
- ^ a b Barregard L (2005). "Mercury from dental amalgam: looking beyond the average". Occup Environ Med. 62 (6): 352–3. doi:10.1136/oem.2004.018911. PMC 1741026. PMID 15901879.
- ^ a b "ATSDR Action Levels for elemental mercury spills" Current Action Level: Executive Summary, p.1; Current MRL: 1.3 Health Guidance Values, p.4; MRL and Action Level definition: Chemical Specific Health Consultation – Mercury, p.5.
- ^ a b "Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects" Dental amalgam mercury exposure: Executive Summary, p.4 and Table 1, p.10; Calculation of inhaled mercury from mercury air concentration: Sample risk characterization, p.31; Absorption of inhaled mercury vapor: Executive Summary, p.4, WHO. 2003.
- ^ a b https://www.fda.gov/cdrh/consumer/amalgams2002.html Archived 11 February 2009 at the Wayback Machine[title missing]
- ^ a b "Safety of dental amalgam. Fédération Dentaire Internationale Technical Report 33". International Dental Journal. 39 (3): 217. 1989. PMID 2793221.
- ^ Rasines Graciela (2008). "Mercury released from amalgam restorations does not give rise to toxic effects on the nervous system of children". Evidence-Based Dentistry. 9 (1): 25–27. doi:10.1038/sj.ebd.6400571. PMID 18364694.
- ^ Rasines Alcaraz, M Graciela; Veitz-Keenan, Analia; Sahrmann, Philipp; Schmidlin, Patrick Roger; Davis, Dell; Iheozor-Ejiofor, Zipporah (2014). "Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth". Cochrane Database of Systematic Reviews (3): CD005620. doi:10.1002/14651858.CD005620.pub2. PMID 24683067.
- ^ Dunsche, A; Kästel, I; Terheyden, H; Springer, IN; Christophers, E; Brasch, J (2003). "Oral lichenoid reactions associated with amalgam: improvement after amalgam removal". The British Journal of Dermatology. 148 (1): 70–6. doi:10.1046/j.1365-2133.2003.04936.x. PMID 12534597. S2CID 39002306.
- ^ "Dental Amalgam: A Scientific Review and Recommended Public Health Service Strategy for Research, Education and Regulation Final Report of the Subcommittee on Risk Management of the Committee to Coordinate Environmental Health and Related Programs". Department of Health and Human Services, Public Health Service. January 1993. Section III. BIOCOMPATIBILlTY OF DENTAL RESTORATIVE MATERIALS. http://web.health.gov/environment/amalgam1/ct.htm Archived 2015-01-07 at the Wayback Machine |accessdate=26 Jan 2015
- ^ Prochazkova, J; Sterzl, I; Kucerova, H; Bartova, J; Stejskal, VD (2004). "The beneficial effect of amalgam replacement on health in patients with autoimmunity" (PDF). Neuro Endocrinology Letters. 25 (3): 211–8. PMID 15349088.
- ^ a b "Reproductive Outcomes among Dental Personnel: A Review of Selected Exposures Journal of the Canadian Dental Association". 2006. Accessed 12 January 2015
- ^ Colson, Dana G. (2012). "A safe protocol for amalgam removal". Journal of Environmental and Public Health. 2012: 517391. doi:10.1155/2012/517391. ISSN 1687-9813. PMC 3270415. PMID 22315627.
- ^ SCENIHR (Scientific Committee on Emerging and NewlyIdentified Health Risks). "Scientific opinion on the Safety of Dental Amalgam and Alternative Dental Restoration Materials for Patients and Users" (PDF). European Commission. Retrieved 6 May 2008.
- ^ Lynch CD; McConnell RJ; Wilson NH (February 2006). "Teaching of posterior composite resin restorations in undergraduate dental schools in Ireland and the United Kingdom". European Journal of Dental Education. 10 (1): 38–43. doi:10.1111/j.1600-0579.2006.00394.x. PMID 16436083.
- ^ Roeters FJ; Opdam NJ; Loomans BA (July 2004). "The amalgam-free dental school". Journal of Dentistry. 32 (5): 371–7. doi:10.1016/j.jdent.2004.02.008. PMID 15193785.
- ^ Mackert, J. Rodway; Wahl, Michael J. (July 2004). "Are there acceptable alternatives to amalgam?". Journal of the California Dental Association. 32 (7): 601–610. doi:10.1080/19424396.2004.12224008. ISSN 1043-2256. PMID 15468542. S2CID 19007625.
- ^ "Vital Link : Mercury Exposure" (PDF). Canadian Association of Neuropathic Doctors. 2010. Archived from the original (PDF) on 13 January 2015. Retrieved 13 June 2015.
- ^ Stein, PS; Sullivan, J; Haubenreich, JE; Osborne, PB (2005). "Composite resin in medicine and dentistry". Journal of Long-Term Effects of Medical Implants. 15 (6): 641–54. doi:10.1615/jlongtermeffmedimplants.v15.i6.70. PMID 16393132.
- ^ Mortensen, ME (1991). "Mysticism and science: the amalgam wars". Journal of Toxicology. Clinical Toxicology. 29 (2): vii–xii. doi:10.3109/15563659109038607. PMID 2051503.
- ^ Eley, BM; Cox, SW (1993). "The release, absorption and possible health effects of mercury from dental amalgam: a review of recent findings". British Dental Journal. 175 (10): 355–62. doi:10.1038/sj.bdj.4808325. PMID 8257645. S2CID 13294444.
- ^ a b "What Patient's Don't Know : Dentist's sweet Tooth for Mercury" (PDF). Mercury Policy Project. 14 February 2006. Archived from the original (PDF) on 10 September 2015. Retrieved 13 June 2015 – via Mpp.cclearn.org.
- ^ Bradbard, Laura (December 1993). "Dental Amalgam: Filling a Need or Foiling Health?". FDA Consumer. 27: 22. Retrieved 29 July 2009.
- ^ "An Uncertain Risk and an Uncertain Future : Assessing the Legal Implications of Mercury Amalgam Fillings". Lsr.nellco.org. Archived from the original (PDF) on 14 February 2009. Retrieved 13 June 2015.
- ^ a b c Fleming, Michael D. (16 February 2007). "Silver-mercury amalgam disclosure and informed consent". Dental Economics. 97 (2). Retrieved 9 May 2020.
- ^ Mandel, ID (1991). "Amalgam hazards. An assessment of research". The Journal of the American Dental Association. 122 (8): 62–5. doi:10.14219/jada.archive.1991.0253. PMID 1918687.
- ^ Berry J, "Lawsuits dismissed: Amalgam rulings are tripartite victory". ADA News 34:3, 23, 2004
- ^ Bellinger, DC; Trachtenberg, F; Barregard, L; Tavares, M; Cernichiari, E; Daniel, D; McKinlay, S (2006). "Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial". JAMA: The Journal of the American Medical Association. 295 (15): 1775–83. doi:10.1001/jama.295.15.1775. PMID 16622139.
- ^ a b Derouen, TA; Martin, MD; Leroux, BG; Townes, BD; Woods, JS; Leitão, J; Castro-caldas, A; Luis, H; et al. (2006). "Neurobehavioral effects of dental amalgam in children: a randomized clinical trial". JAMA: The Journal of the American Medical Association. 295 (15): 1784–92. doi:10.1001/jama.295.15.1784. PMID 16622140.
- ^ Bellinger, DC; Trachtenberg, F; Barregard, L; Tavares, M; Cernichiari, E; Daniel, D; McKinlay, S (2006). "Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial". JAMA: The Journal of the American Medical Association. 295 (15): 1775–83. doi:10.1001/jama.295.15.1775. PMID 16622139.
- ^ "How People are Exposed to Mercury". EPA. 2006. Archived from the original on 30 September 2015.
- ^ Mitchell, Richard J.; Koike, Mari; Okabe, Toru (2007). "Posterior Amalgam Restorations—Usage, Regulation, and Longevity". Dental Clinics of North America. 51 (3): 573–589. doi:10.1016/j.cden.2007.04.004. PMID 17586144.
- ^ "NJDEP-Division of Water Quality- Bureau of Pretreatment and Residuals". New Jersey. Retrieved 12 June 2015.
- ^ "Notice of Dental amalgam Separator Installation" (PDF). Dec.ny.gov. Retrieved 13 June 2015.
- ^ "Michigan Legislature - Section 333.16631". Legislature.mi.gov. Retrieved 12 June 2015.
- ^ McManus, K. R.; Fan, P. L. (August 2003). "Purchasing, operating, and installing dental amalgam separators". The Journal of the American Dental Association. 134 (8): 1054–1065. doi:10.14219/jada.archive.2003.0319. PMID 12956345. S2CID 38241210.
- ^ EPA (2017-06-14). "Effluent Limitations Guidelines and Standards for the Dental Category." Federal Register, 82 FR 27154
- ^ Brinton L (February 1994). "The amalgam controversy". British Dental Journal. 176 (3): 90. doi:10.1038/sj.bdj.4808378. PMID 7599005. S2CID 28081944.
- ^ "New York State Department of Environmental Conservation". Dec.state.ny.us. Retrieved 12 June 2015.
- ^ Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. Concise International Chemical Assessment Document 50. World Health Organization. 2003. Accessed 23 December 2006
- ^ a b c "How Does Mercury Get Into Fish?". Scientific American. 30 December 2011. Retrieved 12 June 2015.
- ^ "Switzerland. United Nations Environment Programme. UNEP Division of Technology, Industry and Economics. Global Mercury Assessment 2013. Geneva, Switzerland: UNEP Chemicals, 2013. PDF. Accessed 23 December 2006" (PDF). Archived from the original (PDF) on 1 April 2014. Retrieved 26 November 2014.
- ^ McManus, Kevin R.; F, P (2003). "Purchasing, installing and operating dental amalgam separators: Practical issues". The Journal of the American Dental Association. 134 (8): 1054–65. doi:10.14219/jada.archive.2003.0319. PMID 12956345. Archived from the original on 23 February 2013.
- ^ "Mercury in the Environment". Usgs.gov. 19 February 2009. Archived from the original on 18 July 2015. Retrieved 12 June 2015.
- ^ "Principles of Ethics and Code of Professional Conduct" (PDF). American Dental Association. Retrieved 13 June 2015.
- ^ "Dental Products Panel". FDA. Retrieved 12 June 2015.
- ^ "Medical Devices Advisory Committee". FDA. Retrieved 12 June 2015.
- ^ a b c "Comments of the American Dental Association before the Dental Products Panel of the Medical Devices Advisory Committee" (PDF). American Dental Association. Archived from the original (PDF) on 16 September 2013. Retrieved 1 April 2013.
- ^ "Dental Amalgam". American Dental Association. Retrieved 5 May 2014.
- ^ Paul L. Powell Jr. (August 2013). "Letters: Amalgam, yes". American Dental Association. Retrieved 5 May 2014.
- ^ Lloyd S. Drucker, D.D.S. (August 2013). "Letters: More on amalgam". American Dental Association. Retrieved 5 May 2014.
- ^ "Amalgam Separators and Waste Best Management". Chicago: American Dental Association. 16 June 2017.
- ^ "Statement on Dental Amalgam". American Dental Association. Retrieved 15 July 2013.
- ^ Clarkson, Thomas. "Current Concepts: The Toxicology of Mercury — Current" (PDF). New England Journal of Medicine. Archived from the original (PDF) on 27 April 2006. Retrieved 1 April 2013.
- ^ a b Maths Berlin, "Mercury in dental-filling materials –– an updated risk analysis in environmental medical terms. An overview of scientific literature published in 1997–2002 and current knowledge." The Dental Material Commission –– Care and Consideration Kv. Spektern, SE–103 33 Stockholm, Sweden. (Final report provided by Regeringskansliet, Government Offices of Sweden) Mercury uptake from amalgam: p.5; Amalgam Elimination from dental care: 6. Environmental medical views of risk management, p.26;
- ^ Food and Drug Administration (3 November 2018). "Appendix I : Summary of Changes to the Classification of Dental Amalgam and Mercury". U.S. Food and Drug Administration. Retrieved 24 June 2021.
- ^ "FDA Issues Final Regulation on Dental Amalgam". FDA. 28 July 2009. Retrieved 1 November 2014.
- ^ "FDA Issues Final Regulation on Dental Amalgam". News and Events. FDA. Retrieved 1 April 2013.
- ^ Health, Center for Devices and Radiological (8 March 2021). "Dental Amalgam Fillings Recommendations - Graphics". FDA.
- ^ "Minamata Convention on Mercury > Convention". Mercuryconvention.org. Archived from the original on 27 March 2014. Retrieved 12 June 2015.
- ^ "Agenda Item 8.6 : Public health impacts of exposure to mercury and mercury compounds: the role of WHO and ministries of public health in the implementation of the Minamata Convention" (PDF). Apps.who.int. Retrieved 13 June 2015.
- ^ "The Minamata Mercury Convention: 12 Things It Does and Doesn't Do". Scientificamerican.com. 10 October 2013. Retrieved 12 June 2015.
- ^ "General Assembly Resolution" (PDF). Fdiworldental.org. September 2010. Retrieved 13 June 2015.
- ^ "Silver Colored Fillings - Amalgam - American Dental Association". Mouthhealthy.org. Retrieved 12 June 2015.
- ^ "OEHHA Proposition 65 Intent to List: Bisphenol A". Oehha.ca.gov. Retrieved 12 June 2015.
- ^ "Dental Amalgam FAQs". Canadian Dental Association. Retrieved 24 November 2014.
- ^ "Safety of Dental Amalgam". Policy Statement 6.18 Australian Dental Association Inc. April 2012. Accessed 8 January 2014
- ^ "The safety of dental amalgam and alternative dental restoration materials for patients and users." Scientific Committee on Emerging and Newly Identified Health Risks SCENIHR, European Commission, Health & Consumer Protection Directorate-General. 6 May 2008. Accessed 8 January 2014
- ^ "British Dental Health Foundation Policy Statement Dental Amalgam" (PDF). Dentalhealth.org. Archived from the original (PDF) on 13 January 2015. Retrieved 13 June 2015.
- ^ a b c d e f "Review of Norwegian experiences with the phase-out of dental amalgam use", TA 2946, Climate and Pollution Agency, 2012. Accessed 8 January 2014
- ^ a b "The Mercury Problem: Reducing and eliminating mercury pollution in Norway 2010". Accessed 8 January 2014
- ^ http://www.government.se/sb/d/11459/a/118550 Archived 24 September 2014 at the Wayback Machine[title missing]
- ^ "IAOMT Achievements" (PDF). 10 March 2016. Retrieved 13 June 2015.
- ^ http://www.kemi.se/upload/Trycksaker/Pdf/Rapporter/Rapport4_04.pdf Archived 20 December 2005 at the Wayback Machine[title missing]
- ^ a b http://www.government.se/sb/d/2969/a/223295 Archived 23 December 2014 at the Wayback Machine[title missing]
- ^ a b "Questions and answers: EU mercury policy and the ratification of the Minamata Convention" (Press release). European Commission. Retrieved 3 January 2018.
- ^ "Dental amalgam". British Dental Association. Retrieved 3 January 2018.
- ^ Bailey, O; Vernazza, CR; Stone, S; Ternent, L; Roche, A-G; Lynch, C (2020). "Amalgam phase-down part 1: UK-based posterior restorative material and technique use". JDR Clinical & Translational Research. 7 (1): 41–49. doi:10.1177/2380084420978653. PMC 8674792. PMID 33300416.
- ^ Bailey, O; Vernazza, C; Stone, S; Ternent, L; Roche, A-G; Lynch, C (2020). "Amalgam phase-down part 2: UK-based knowledge, opinions and confidence in the alternatives". JDR Clinical & Translational Research. 7 (1): 50–60. doi:10.1177/2380084420954766. PMC 8674793. PMID 33300424.
- ^ "アマルガム除去の詳細" (in Japanese). 日本橋中央歯科. Retrieved 12 October 2023.
External links edit
- Mercury Policy Project
- Position Statement against Dental Mercury Amalgam Fillings for Medical and Dental Practitioners, Dental Students, and Patients, International Academy of Oral Medicine and Toxicology (IAOMT), 16 April 2013 Archived 31 March 2016 at the Wayback Machine
- The "Mercury Toxicity" Scam: How Anti-Amalgamists Swindle People