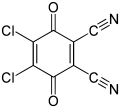
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols,[3] phenols,[4] and steroid ketones.[5] DDQ decomposes in water, but is stable in aqueous mineral acid.[6]
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4,5-Dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile[2] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | DDQ | ||
| ChemSpider | |||
| ECHA InfoCard | 100.001.402 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8Cl2N2O2 | |||
| Molar mass | 227.00 g·mol−1 | ||
| Appearance | yellow to orange powder | ||
| Density | 1.7g/cm3 | ||
| Melting point | 210–215 °C (410–419 °F; 483–488 K) (decomposes) | ||
| Boiling point | 301.8 °C (575.2 °F; 575.0 K) at 760mmHg | ||
| reacts | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H301 | |||
| P264, P270, P301+P310, P321, P330, P405, P501 | |||
| Flash point | 136.3 °C (277.3 °F; 409.4 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Preparation edit
Synthesis of DDQ involves cyanation of chloranil. J. Thiele and F. Günther first reported a 6-step preparation in 1906.[7] The substance did not receive interest until its potential as a dehydrogenation agent was discovered. A single-step chlorination from 2,3-dicyanohydroquinone was reported in 1965.[8]
Reactions edit
The reagent removes pairs of H atoms from organic molecules. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene:
- 2 C6Cl2(CN)2O2 + C10H12 → 2 C6Cl2(CN)2(OH)2 + C10H8
The resulting hydroquinone is poorly soluble in typical reaction solvents (dioxane, benzene, alkanes), which facilitates workup.
Solutions of DDQ in benzene are red, due to the formation of a charge-transfer complex.[9]
Dehydrogenation edit
Aromatization edit
Cross-Dehydrogenative Coupling edit
Safety edit
DDQ reacts with water to release highly toxic hydrogen cyanide (HCN).[6]
References edit
- ^ 2,3-Dichloro-5,6-dicyano-p-benzoquinone at Sigma-Aldrich
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 50. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Braude, E. A.; Linstead, R. P.; Wooldridge, K. R. H. (1956). "593. Hydrogen Transfer. Part IX The Selective Dehydrogenation of Unsaturated Alcohols by High-potential Quinones". Journal of the Chemical Society (Resumed). 1956: 3070–3074. doi:10.1039/JR9560003070.
- ^ Becker, H. D. (1965). "Quinone Dehydrogenation. I. Oxidation of Monohydric Phenols". Journal of Organic Chemistry. 30 (4): 982–989. doi:10.1021/jo01015a006.
- ^ Turner, A. B.; Ringold, H. J. (1967). "Applications of High-potential Quinones. Part I. The Mechanism of Dehydrogenation of Steroidal Ketones by 2,3-Dichloro-5,6-Dicyanobenzoquinone". Journal of the Chemical Society C: Organic. 1967: 1720–1730. doi:10.1039/J39670001720.
- ^ a b Buckle, Derek R.; Collier, Steven J.; McLaws, Mark D. (2005). "2,3-Dichloro-5,6-dicyano-1,4-benzoquinone". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd114.pub2. ISBN 0471936235.
- ^ Thiele, J.; Günther, F. (1906). "Ueber Abkömmlinge des Dicyanhydrochinons". Justus Liebig's Annalen der Chemie. 349 (1): 45–66. doi:10.1002/jlac.19063490103.
- ^ Walker, D.; Waugh, T. D. (1965). "2,3-Dichloro-5,6-Dicyanobenzoquinone (DDQ). A New Preparation". The Journal of Organic Chemistry. 30 (9): 3240. doi:10.1021/jo01020a529.
- ^ Rathore, Rajendra; Kochi, Jay K. (2000), "Donor/acceptor organizations and the electron-transfer paradigm for organic reactivity", Advances in Physical Organic Chemistry, Elsevier, pp. 193–318, doi:10.1016/s0065-3160(00)35014-6, ISBN 9780120335350
- ^ Brown, W.; Turner, A. B. (1971). "Application of High-potential Quinones. 7. Synthesis of Steroidal Phenanthrenes by Double Methyl Migration". Journal of the Chemical Society C: Organic. 1971: 2566–2572. doi:10.1039/J39710002566. PMID 5167256.
- ^ Zhang, Y.; Li, C. J. (2006). "DDQ-Mediated Direct Cross-Dehydrogenative-Coupling (CDC) between Benzyl Ethers and Simple Ketones". Journal of the American Chemical Society. 128 (13): 4242–4243. doi:10.1021/ja060050p. PMID 16568995.