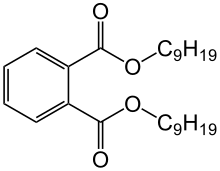
Diisononyl phthalate (DINP) is a phthalate used as a plasticizer. DINP is typically a mixture of chemical compounds consisting of various isononyl esters of phthalic acid, and is commonly used in a large variety of plastic items.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Benzenedicarboxylic acid, di-C8-10 branched alkyl esters, C9 rich | |
| Other names
1,2-Benzenedicarboxylic acid, 1,2-diisononyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | DINP |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.044.602 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C26H42O4 | |
| Molar mass | 418.618 g·mol−1 |
| Appearance | Oily viscous liquid |
| Density | 0.98 g/cm3 |
| Melting point | −43 °C (−45 °F; 230 K) |
| Boiling point | 244 to 252 °C (471 to 486 °F; 517 to 525 K) at 0.7 kPa |
| <0.01 g/mL at 20 °C | |
| Viscosity | 64 to 265 mPa·s |
| Hazards | |
| Flash point | 221 °C (430 °F; 494 K) (c.c.) |
| 380 °C (716 °F; 653 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Health Issues edit
The European Union has set a maximum specific migration limit (SML) from food contact materials of 9 mg/kg food for the sum of diisononyl phthalates and diisodecyl phthalates.[2]
DINP is listed as a substance "known to the State of California to cause cancer" under Proposition 65 legislation.[3]
Studies find that exposure to environmentally relevant concentrations of DINP in zebrafish disrupt the endocannabinoid system (ECS) and affect reproduction in a gender specific manner,[4] and have other adverse effects on aquatic organisms, as DINP upregulates orexigenic signals and causes hepatosteatosis together with deregulation of the peripheral ECS and lipid metabolism.[5]
ECHA's Risk Assessment Committee (RAC) has concluded, on March 7, 2018, that Di-isononyl phthalate (DINP) does not warrant classification for reprotoxic effects under the EU's Classification, Labelling and Packaging (CLP) regulation [6]
See also edit
References edit
- ^ Diisononyl phthalate at Inchem.org
- ^ "EU legislative list for food contact materials".
- ^ "State of California, Chemicals known to the state to cause cancer or reproductive toxicity, January 3, 2014" (PDF). Archived from the original (PDF) on 2014-01-10.
- ^ Forner-Piquer, Isabel; Santangeli, Stefania; Maradonna, Francesca; Rabbito, Alessandro; Piscitelli, Fabiana; Habibi, Hamid R.; Di Marzo, Vincenzo; Carnevali, Oliana (2018-10-01). "Disruption of the gonadal endocannabinoid system in zebrafish exposed to diisononyl phthalate". Environmental Pollution. 241: 1–8. doi:10.1016/j.envpol.2018.05.007. ISSN 0269-7491. PMID 29793103. S2CID 44120848.
- ^ Forner-Piquer, Isabel; Maradonna, Francesca; Gioacchini, Giorgia; Santangeli, Stefania; Allarà, Marco; Piscitelli, Fabiana; Habibi, Hamid R; Di Marzo, Vincenzo; Carnevali, Oliana (2017-08-14). "Dose-Specific Effects of Di-Isononyl Phthalate on the Endocannabinoid System and on Liver of Female Zebrafish". Endocrinology. 158 (10): 3462–3476. doi:10.1210/en.2017-00458. ISSN 0013-7227. PMID 28938452.
- ^ "Evaluation of new scientific evidence concerning DINP and DIDP". European Chemicals Agency. Archived from the original on 2022-09-01. Retrieved 2020-01-26.